2018 H2 Chemistry JC students post your questions here
-
Originally posted by UltimaOnline:
The following MCQ is a Cambridge A level MCQ, used in both the UK and Singapore papers.
When iron reacts with aqueous iron(III) ions, iron(II) ions are formed as the only product. A final mixture, after the reaction has taken place, contains equal numbers of moles of Fe2+(aq) and Fe3+(aq). Assuming the reaction has gone to completion, how many moles of Fe(s) and Fe3+(aq) were in the starting mixture?
I remember coming across a Singapore JC Prelim / Tutorial MCQ which used this MCQ and gave it a twist to make it tougher. I searched for it but can't find it. If anyone recognizes the Singapore JC Prelim / Tutorial MCQ I'm referring to, please post the JC and year, or the MCQ itself, thanks.
The modified Prelim / Tutorial MCQ could be that the final amount of Fe2+(aq) and Fe3+(aq) were in the molar ratio of 2:1 or 1:2 (or any ratio you like), instead of 1:1. But such a slightly modified MCQ would still be considered easy (to be honest).I seem to recall the modified Prelim / Tutorial MCQ being a tad more interesting (students would say 'sadistic') than this, but a pity I forget the exact modification. Anyone recognizes the modified Prelim / Tutorial MCQ I'm referring to, please share it here, thanks.
-
Came across this in a 2017 Singapore JC Prelim Paper, which shall remain unnamed.
Marker’s comment : There are still some students who drew mechanism like A --> B --> C --> D, the correct way to draw is A --> B, B --> C, C --> D.
No, that is just totally wrong. Although Cambridge will accept either way for A level purposes, the correct (ie. University) way, and indeed the way preferred by Cambridge markers at A levels, is to draw A --> B --> C --> D.
To draw A --> B, B --> C, C --> D is a terribly inefficient and childish way, which Cambridge tolerates but discourages, even at A levels.
Here's an example of how we draw mechanisms at University levels, and thus also the recommended way for competent (not childish) A level students :

-
[Singapore] - A young woman scholar from the Agency for Science, Technology and Research (A*Star) was suspected to have taken her own life at a laboratory early Tuesday (Jan 16 2018) morning. Colleagues of Katarina Chlebikova, working at the Institute of Molecular and Cell Biology (IMCB), said that the 26-year-old Slovakian had been troubled over work and relationship issues before her death. In response to media queries, the police said that they were alerted to the case at 10.44am on Tuesday. Her body was found on the eighth storey of 61 Biopolis Drive, where the institute is located. “A 26-year-old woman was found motionless and was pronounced dead by paramedics at scene. Police are investigating the unnatural death,� they added. TODAY understands that Chlebikova left behind a note suggesting that she killed herself via nitrogen poisoning.
http://www.todayonline.com/singapore/astar-scholar-found-dead-lab
BedokFunland JC's comments on the chemistry involved :
Nitrogen gas itself is physiologically inert and non-toxic, unlike carbon monoxide. However, deliberately pumping into a room excess nitrogen gas, displaces all other gases from the room, including oxygen. This results in asphyxiation and death from oxygen deprivation, as would occur with carbon monoxide poisoning.
That this method can be used, and has been used, to carry out covert assassinations, is known to agents of intelligence agencies such as the CIA, Mossad, MI6, KGB, etc. In the case of the 26-year-old Slovakian young woman in Singapore, she used this method for committing suicide.
Spiritually and karmically, the state of emotional and psychological distress that drove her to commit suicide, will accompany her into the afterlife, and will pose some difficulty in releasing her consciousness from her post-mortem emotional pain and psychosis, and she may need some time to herself before willing to accept and experience love and forgiveness, for sufficient healing of her psychological trauma to occur, before she can move forward vibrationally, onto the intermissive period proper, where further healing, life review and preparation for her next physical incarnation can occur. She will be supported by her guides & helpers at every step of the way, even if she may not be aware of it through her emotional pain. Rest in peace now, Miss Katarina Chlebikova.
-
[Chemistry / Medicine / Biology] - YouTube account 'Chubbyemu', a medical doctor, did an 're-enactment' video on the medical tragedy of chemist Karen Wetterhahn

https://www.youtube.com/watch?v=NJ7M01jV058

-
05 Feb 2018 : Updated with a new BedokFunland JC original H2 Chem Qn on Adrenaline.
-
[Medicine] - Stanford University's revolutionary approach of using the body's own immune system against the cancer, works “startlingly well�, where all other methods (eg. chemotherapy, radiation therapy) have failed.
Scientists at Stanford University in the US found that injecting tiny amounts of two drugs directly into a tumour not only kills the original cancer, but also triggers an “amazing body-wide� immune reaction which destroys distant cancer cells and all metastatic tumors throughout the entire body.
90% of advanced-stage cancer-ridden mice were completely cured of cancer after a single injection, and 100% of the mice were completely cured of cancer after a 2nd injection.
With this breakthrough approach, the elusive cure for cancer is much closer within reach than ever before. 2018 could be the year in which humanity finds the cure for cancer (assuming big pharma doesn't get to the Stanford researchers first).
http://www.theweek.co.uk/91359/cancer-vaccine-to-enter-human-trials
http://www.dailymail.co.uk/health/article-5337727/Human-trials-start-cancer-vaccine.html
http://www.sciencemag.org/news/2018/01/injection-helps-immune-system-obliterate-tumors-least-mice
-
Chemical in McDonald’s fries may be the cure for baldness, study finds
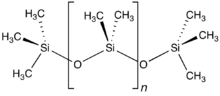
For decades, McDonalds has been quietly adding a chemical Polydimethylsiloxane (PDMS) into the oil used for cooking French fries, simply because PDMS reduces oil splattering at high temperatures, making French fries easier to cook.
Now, a research team from Japan have found that PDMS helps hair grow on mice, and they expect the findings to be applicable to human beings as well.
Who knows what else McDonalds or other giant F&B brands, have been adding to our foods too all these years without a full understanding of their biochemical effects, eh?
-
I love chem
-
Many Singapore JCs are using this fun calculations + bonding question to tekan their J1s :
When equal moles of Xe and F2 are reacted, a solid product is generated. Given the final gaseous pressure is 70% of the initial gaseous pressure, and that the unreacted Xe and F2 are in a 4:3 molar ratio, calculate the formula of the solid product, and draw its structure, using stereochemical formula (ie. wedge-dash diagram).
-
A 2018 Original BedokFunland JC H2 Chemistry Challenge
Compare and explain the relative acidities of the ortho, para and meta positional isomers of nitrophenol.
-
I love chem
-
i love chem
-
A 2018 Original BedokFunland JC H2 Chemistry Challenge
Draw the curved-arrow electron-flow reaction mechanisms (in both directions) to explain how pH shifts the position of equilibrium between the vanadate(V) ion and the oxovanadium(V) ion.
-

https://en.wikipedia.org/wiki/Formaldehyde
Due to a horrific medical error, a poor Russian woman was ’embalmed alive’, receiving an intravenous infusion of toxic formaldehyde (ie. methanal in H2 Chem, it's so toxic that not even bacteria and fungi decomposers can survive, hence its use as an embalming fluid to permanently preserve dead human bodies) by error, instead of harmless sodium chloride solution. She violently convulsed non-stop, screaming in excruciating agony for 14 hours before dying.


-
For every 1 such case reported, there are 999 cases that go unreported, all around the world.
[Thailand] - British woman drugged unconscious and raped.

http://www.asiaone.com/asia/death-island-koh-tao-news-again-after-rape-claim-british-woman

https://www.youtube.com/results?search_query=roofies+date+rape+spike+drink+social+experiment

-
Indonesia steamrolls bootleg booze as death toll nears 100

http://www.asiaone.com/asia/indonesia-steamrolls-bootleg-booze-death-toll-nears-100
[Medicine / Chemistry / Biology] - Why is methanol toxic? (eg. even if you don't die, you will become permanently blind in both eyes, after consuming alcoholic drinks adulterated with cheap toxic methanol, sold by profit-seeking criminal syndicates trying to circumvent the heavy tax on drinkable ethanol imposed by governments)

https://en.wikipedia.org/wiki/Methanol_toxicity#Signs_and_symptoms
-
About Mole and Stoichiometry - Titration part
1) Currently learning it - I get confused when tackling the tiration questions when practicing. Like what do I do? What are the steps? Like I know there's a few different kinds of titration (back, acid-base etc.), what are the difference in between them or am I required to know the differences?
Btw, learning it as private candidate and using CS toh guide books. However my version of CS toh Chemistry Study guide (Edition 3.0) doesn't has the titration part included.
2) Also just want to check, when computing the oxidation states of the terms in the equation, all group 3 elements are all +3. So when they meant group 3 elements, are they referring to the scandium column?
-
Originally posted by JustUrAvgSingaporean:
About Mole and Stoichiometry - Titration part
1) Currently learning it - I get confused when tackling the tiration questions when practicing. Like what do I do? What are the steps? Like I know there's a few different kinds of titration (back, acid-base etc.), what are the difference in between them or am I required to know the differences?
Btw, learning it as private candidate and using CS toh guide books. However my version of CS toh Chemistry Study guide (Edition 3.0) doesn't has the titration part included.
2) Also just want to check, when computing the oxidation states of the terms in the equation, all group 3 elements are all +3. So when they meant group 3 elements, are they referring to the scandium column?
Q1. For H1, no practical paper, no worries. Whether acid-base or redox titrations, just deal accordingly to the question.Q2. Group III = Group 13, so yes Group 3 refers to Sc, Y, etc.


