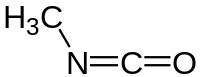CIE QUESTIONS
-
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s13_qp_11.pdf
white ppt formed in first reaction that may be AgCl or AgBr or AgI or AgAt.
ppt dissolve in concentrated aqueous ammonia means it is either Cl- or Br-.
But on addition of HNO3 the ppt dissolves....Can you explain what happens here? and why Cl- is present here not Br-? Has this something to do with dynamic equilibrium?
-
Originally posted by hoay:
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s13_qp_11.pdf
white ppt formed in first reaction that may be AgCl or AgBr or AgI or AgAt.
ppt dissolve in concentrated aqueous ammonia means it is either Cl- or Br-.
But on addition of HNO3 the ppt dissolves....Can you explain what happens here? and why Cl- is present here not Br-? Has this something to do with dynamic equilibrium?
For Q17, since the ppt dissolved in dilute NH3(aq), the ppt must have been AgCl, which re-precipitated out upon addition of acid, because position of equilibrium shifts right : NH3 ligands <~> NH3 (aq) <~> NH4+(aq)To address your question directly, it's Cl- not Br-, because the ppt dissolved in *DILUTE* NH3(aq). If the question used concentrated aqueous ammonia, then both Cl- and Br- could be possible (ie. then the MCQ would be flawed and cannot be answered definitively, unless the color of the ppt was specified).
-
thank you so much....this question disturbed me for so long.
-
Originally posted by hoay:
thank you so much....this question disturbed me for so long.
No prob, hoay! :) -
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s15_qp_13.pdf
Q.8 Why C is not the answer? As the [methanol] will be become constant when the reaction is complete.
Q.28 What is X in this diagram?
-
Originally posted by hoay:
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s15_qp_13.pdf
Q.8 Why C is not the answer? As the [methanol] will be become constant when the reaction is complete.
Q.28 What is X in this diagram?
Q8. Option C assumes that the basic hydrolysis of ester is a 0 order reaction, but it is in fact, a 2nd order reaction. Hence the correct answer is option A. Option B is characteristic of an auto-catalytic reaction, but neither product of the basic hydrolysis catalyzes the hydrolysis. Option D is just nonsense.Q28. X is the transition state as the C-Br bond is being broken, ie. a C-Br partial bond, a partial +ve charge on the C atom, and a partial -ve charge on the Br atom.
-
Order of the reaction is not taught at first year of A-level in CIE. any other explanation?
-
Another query is from June 2016 /MCQ. I am afraid that this paper is not available in any site on the web.
Methyl isocyanate , CH3NCO, is a toxic liquid.
What is the approximate angle between the bonds formed by the N atoms in a a molecule of methyl isocyanate?
H3C- N=C=O 120 is the answer.
The electron pairs around N are 3 ; there are 2 bond pairsaround N; one is lone pairs. 321....so the bond angle must deviate from the ideal situation 330...120. It would be less than 120, around 109.
-
Originally posted by hoay:
Order of the reaction is not taught at first year of A-level in CIE. any other explanation?
As reaction progresses, [ester] decreases, hence rate of reaction decreases, hence you obtain the decreasing rate curve in option A. -
Originally posted by hoay:
Another query is from June 2016 /MCQ. I am afraid that this paper is not available in any site on the web.
Methyl isocyanate , CH3NCO, is a toxic liquid.
What is the approximate angle between the bonds formed by the N atoms in a a molecule of methyl isocyanate?
H3C- N=C=O 120 is the answer.
The electron pairs around N are 3 ; there are 2 bond pairsaround N; one is lone pairs. 321....so the bond angle must deviate from the ideal situation 330...120. It would be less than 120, around 109.
Excellent question. There are actually 5 levels to this at-1st-glance straightforward question.At the simplest level, the N atom has 3 electron regions or charge clouds : a single bond, a double bond, and a lone pair. Hence electron geometry is expected to be trigonal planar, ie. bond angle close to 120 degrees.
On a deeper level, the lone pair is expected to have slightly more repelling power, hence the bond angle may now be expected to decrease slightly below 120 degrees.
On an even deeper level, the double bond is expected to have slightly more repelling power, hence the bond angle may now be expected to increase slightly back to approximately 120 degrees.
On a way deeper level, O has the greatest electronegativity, hence the O atom withdraws strongly by induction (ie. through the sigma bond) as well as by resonance (ie. through the pi bond) from the C atom, which consequently itself becomes somewhat electron-withdrawing vis-Ã -vis the N atom, despite N's greater electronegativity vis-Ã -vis the C atom. The effect of which is to reduce the electron density of the N=C double bond, hence the bond angle may now be expected to decrease back to slightly less than 120 degrees.
On a way, way, way deeper level, you should be able to figure out that there is a minor resonance contributor in which the lone pair on the N atom forms a 2nd pi bond with the C atom, and the pi bond between the C atom and the O atom becomes a lone pair on the O atom, ie. +ve formal charge on the N atom, -ve formal charge on the O atom. This is a minor resonance contributor, valid only because of the high electronegativity of O. As such, the lone pair resides in an orbital which is mostly sp2, but has a little unhybridized 'p' character, in order for the slight overlap of unhybridized p orbitals (of N and C) to occur and for slight delocalization of the lone pair to form a partial 2nd pi bond with the C atom, ie. the N=C bond has partial triple bond character in the resonance hybrid. As such, the bond angle may now be expected to increase to above 120 degrees, ie. the N atom is mostly sp2 hybridized, with a little sp hybridized character.
Indeed, experimental evidence has proven the C-N-C bond angle to be approximately 125 degrees, thus concurring with my 5 points of consideration above.
Of course, Cambridge is aware that A level students are not able to consider all these factors to elucidate as deeply as I just did, and hence if it were a non-MCQ question, Cambridge will be reasonable and accept reasonable answers on condition of reasonable rationales. For a MCQ question, Cambridge will (as they did in this MCQ) only provide clear-cut options such as A) 120 degrees B) 109.5 degrees C) 90 degrees D) 180 degrees, in which the required answer is obvious, because the A level student is expected to choose the option which gives the closest answer.
-
tahnk you for Excellent explanation
-
Originally posted by hoay:
tahnk you for Excellent explanation
No prob, Hoay! :) -
For the interest of those of you following this thread on CIE papers, one of the toughest questions Cambridge has ever set, is Q6 f ii & iii of the CIE A Level 2013 Nov Question Paper 43. Go check it out and have fun! ;D
-
Another delightfully tricky IQ-type question that Cambridge can be proud of, would be Q5a of the CIE A Level 2009 Nov Question Paper 42.
I recall someone (was it Hoay?) asking me about this question on the forum some time back.
-
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s14_qp_21.pdf
qn 4eiii
why does chloroethane undergo substitution reaction with NaCN(given in qn)? i thought usually KCN is used for nucleophilic sub rxns?
-
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s14_qp_22.pdf
qn 5b when the qn states , give the structures, can i draw out the structures instead of writing down the structural formula? for example, in general, can i draw https://www.google.com.sg/search?q=propanoic+acid&source=lnms&tbm=isch&sa=X&ved=0ahUKEwi7lYC4rIvUAhXDMI8KHTecC24Q_AUICigB&biw=1047&bih=559#imgrc=Zz9g0od4Nzy2qM:
instead of writing ch3ch2co2h
i feel that drawing out the structure is easier as i tend to get confused when writing down the structural formula in a single line.
-
Originally posted by glitter58:
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s14_qp_21.pdf
qn 4eiii
why does chloroethane undergo substitution reaction with NaCN(given in qn)? i thought usually KCN is used for nucleophilic sub rxns?
In future pls include the links to the mark schemes (on same website), to facilitate convenience.The counter cation is spectatory hence irrelevant, it's the CN- anion nucleophile which is participatory and reacts. Beyond A levels, there are other considerations why a particular counter cation may be preferred, even if it's only spectatory, but these are not relevant for A levels.
When Cambridge simply asks for "structural formula", they will accept any of the 3 types : displayed or full, skeletal, or condensed. It's only when Cambridge specifies 1 of the 3 types, then only that type of structural formula is accepted. Otherwise, any of the 3, including a hybrid of the 3, will be accepted.
-
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s14_qp_41.pdf
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s14_ms_41.pdf
qn 3biii) what is the significance of the word excess in the qn? will the product be different if kmno4 is not used in excess?
4a) when asked to indetify functional group, is benzene a functional group?
4bii) for drawing the structure, in the mark scheme, isnt Br in the 3rd position with regards to one of the OH groups? (altho the br in the 2nd position with regards to the other oh group) but i thought OH group is 2 and 4 directing so why is the bromine substitued there?
-
Originally posted by glitter58:
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s14_qp_41.pdf
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s14_ms_41.pdf
qn 3biii) what is the significance of the word excess in the qn? will the product be different if kmno4 is not used in excess?
4a) when asked to indetify functional group, is benzene a functional group?
4bii) for drawing the structure, in the mark scheme, isnt Br in the 3rd position with regards to one of the OH groups? (altho the br in the 2nd position with regards to the other oh group) but i thought OH group is 2 and 4 directing so why is the bromine substitued there?
Q3) Excess is just to play safe that the required answer will be the completely oxidized product (because there are several partially oxidized intermediates possible, since there are 3 functional groups to be oxidized per molecule here), to ensure complete oxidation of benzene side chain *and* the 2 alkene groups, hence *excess* KMnO4 (ie. mole ratio >> 1:1, in fact, the actual stoichiometry required is > 3:1, because to fully oxidize *each* of the 3 functional groups will require more than 1 mole of KMnO4, but almost all Singapore A level students will die if Cambridge asks *exactly* how many moles required in total) is required to generate the fully oxidized product. Otherwise, if 'excess' wasn't specified by Cambridge, some students may be confused and imagine that KMnO4 might be limiting, and these students will have no idea which of the 3 functional groups gets priority to be oxidized, and oxidized to which extents.
Q4a) Yes it is.
Q4b) *Each* phenolic OH group is activating *and* ortho-para directing. Since there are 2 phenolic OH groups adjacent to each other, hence with excess Br2(aq) (instead of Br2 in CCl4), all 3 available positions may be brominated (note the Cambridge Mark Scheme is reasonable, unlike Singapore JC mark schemes which are anal, and Cambridge accepts either 2 or 3 Br atoms substituted), even after considering the deactivating effects of the aldehyde group (which is why after considering both activating and deactivating effects, Cambridge accepts either 2 or 3 Br atoms substituted in, although the better answer is still 3 Br atoms substituted in, all factors considered).
-
In an experiment, a sample of a pure gas is put into a syringe at a temperature of 300Kand pressure of 16kPa. The gas is compressed until the volume occupied by the gas is halved.
After compression the temperature of the gas in the syringe is 373K and the pressure is 40kPa.
Which statement is correct?
A Intermolecular forces between the gas molecules are significant.
B It is possible to calculate the number of moels of gas present using these data alone.
C the gas is behaving ideally.
D the pressure used are too high for ideal has behaviour.
I did not understanf this behaviour by the gas. The pressure is increasing from 16 kPa to 40kPa, this means that molecules are going far apart it means ideal behaviour. But then Temperature also increases from 300K to 373K....What is happeneing?
-
Originally posted by hoay:
In an experiment, a sample of a pure gas is put into a syringe at a temperature of 300Kand pressure of 16kPa. The gas is compressed until the volume occupied by the gas is halved.
After compression the temperature of the gas in the syringe is 373K and the pressure is 40kPa.
Which statement is correct?
A Intermolecular forces between the gas molecules are significant.
B It is possible to calculate the number of moels of gas present using these data alone.
C the gas is behaving ideally.
D the pressure used are too high for ideal has behaviour.
I did not understanf this behaviour by the gas. The pressure is increasing from 16 kPa to 40kPa, this means that molecules are going far apart it means ideal behaviour. But then Temperature also increases from 300K to 373K....What is happeneing?
The formation of significant intermolecular attractions (eg. van der Waals forces or hydrogen bonds) between the gas molecules when the gas is compressed (ie. molecules brought closer together) is exothermic, resulting in an increase in temperature. Hence, option A is the answer. -
But the answer is C
-
Originally posted by hoay:
But the answer is C
Can you post the link to the CIE mark scheme? -
Before compression : PV = nRT, nR = (16000)(2 vol) / (300)
After compression : PV = nRT, nR = (40000)(1 vol) / (373)
If gas behaves ideally, then nR before compression, divided by nR after compression, will be 1.00, which is not the case if you do the division. Hence, the gas is not behaving ideally.
-
Sure this is the link for Question paper, MS.
http://pastpapers.papacambridge.com/view.php?id=Cambridge%20International%20Examinations%20%28CIE%29/AS%20and%20A%20Level/Chemistry%20%289701%29/2016%20Nov/9701_w16_qp_12.pdf
http://pastpapers.papacambridge.com/view.php?id=Cambridge%20International%20Examinations%20%28CIE%29/AS%20and%20A%20Level/Chemistry%20%289701%29/2016%20Nov/9701_w16_ms_12.pdf
The examiners' report for this question states:
29% of candidates chose the correct answer C. The mst commonly chosen answer was D chosen by 29% of candidates. If a fixed mass of gas is behaving ideally the sum PV/RT gives a constant value. for both the conditions given in the question
PV/RT =6.418 (if volume of 1dm3 and 0.5 dm3 are assumed. therefore gas is behaving ideally.
the paper is NOV 2016/12/Q.8