CO2 has same m.p and bp
-
CO2 has same melting and boiling point. Is is due to the structure and non-polar nature?
-
Originally posted by hoay:
CO2 has same melting and boiling point. Is is due to the structure and non-polar nature?
Yes, the fact that CO2 is overall non-polar, yet possesses 2 significant individual dipoles in a linear molecular geometry, results in its sublimative property (ie. only existing at solid and gaseous states) at 1 bar or 1 atm. The liquid state is only possible at significantly higher pressures, as illustrated in its phase diagram.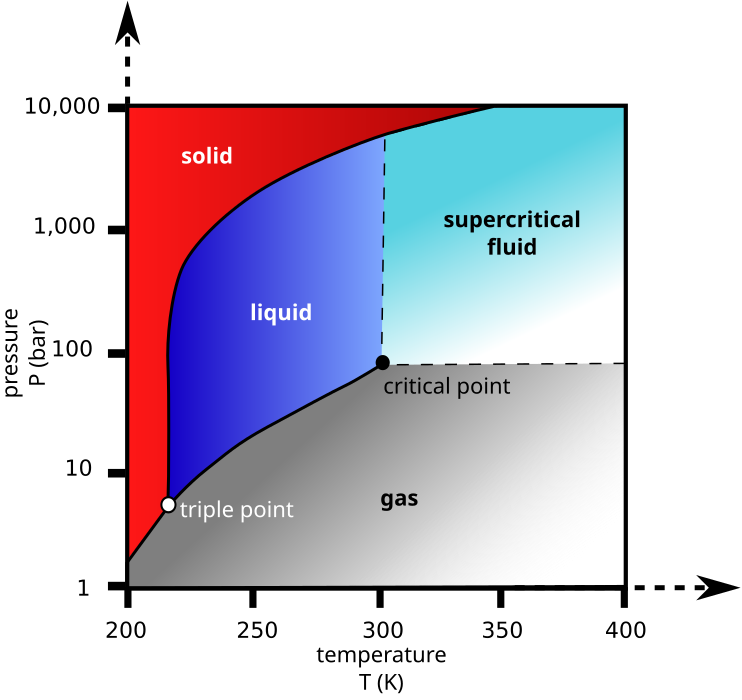
Image credits : Wikipedia
-
thank you