2017 H2 Chemistry JC1 & 2 students post your questions here
-
^_^
-
Hi! What is meant by "vacant low-lying orbital" for dative covalent bond?
-
Also I am confused about where to label the bond angles for 3D structures. For example the picture in the link below shows 2 different ways to label. Which one is correct and how do I know where to label the bond angle? :)
http://imgur.com/0KfJUVE
-
Originally posted by flowerd:
Hi! What is meant by "vacant low-lying orbital" for dative covalent bond?
Vacant means empty, unoccupied by electrons.Low-lying refers to being energetically accessible by that element.
Period 1 elements : only 1s orbital is low-lying.
Period 2 elements : 2s orbital, and 2px, 2py and 2pz orbitals, are all low-lying.
Period 3 elements : 3s orbital, and 3px, 3py, 3pz orbitals, and 3d(x2-y2), 3d(z2), 3d(xy), 3d(xz), 3d(yz) orbitals, are all low-lying.
A Lewis acid or electrophile can only accept a dative covalent bond pair of electrons into one of its vacant low-lying orbital (of the acceptor atom).
-
Originally posted by flowerd:
Also I am confused about where to label the bond angles for 3D structures. For example the picture in the link below shows 2 different ways to label. Which one is correct and how do I know where to label the bond angle? :)
http://imgur.com/0KfJUVE
Both are correct and acceptable by Cambridge, because all the bond angles in the tetrahedral molecular geometry are all equally 109.5 degrees. -
Flowerd, you're a JC1 student who is trying out JC life without tuition, yes?
So did you understand my replies to your questions? Have they cleared your doubts?
All JC students, feel free (it's literally free) to register an SgForums account to post your H2 / H1 Chem questions in this thread.
-

-
Note for H2 Chemistry : it is a non-issue, because Cambridge only cares that you write the symbol for it (ie. 298K), and not spell it out (eg. 298 Kelvins / 298 kelvins / 298 Kelvin / 298 kelvin).
Are there reasons for the discrepancies in absolute temp units - Kelvin vs. kelvins vs. degrees Kelvin?
Before 1968, the units for absolute temperature were described as "degrees Kelvin" or "degrees absolute." After that, the SI system got rid of the idea of "degree" for absolute temperature, so the new unit should apparently be expressed as a "kelvin" (with lowercase k) and abbreviated simply "K" (without the degree sign). Also, official SI conventions suggest that not only should the unit name be lowercase, but it should be pluralized as other units would be: "Il en résulte que la température thermodynamique du point triple de l’eau est égale à 273,16 kelvins exactement, Ttpw = 273,16 K." Or, in English: "It follows that the thermodynamic temperature of the triple point of water is exactly 273.16 kelvins, Ttpw = 273.16 K."
Despite the official SI usage, however, it seems that there are still a variety of conventions in use. Many of the questions on this forum, for example, use Kelvin (with a capital) instead of kelvin in referencing the unit. Also, it appears that the plural usage is somewhat mixed in the physics literature: something like "200 kelvins" occurs, but more rarely than "200 kelvin" or even "200 Kelvin." The NIST guidelines do not list the kelvin as an exception to the normal pluralization rules: "the following plurals are irregular: Singular — lux, hertz, siemens; Plural — lux, hertz, siemens." On the other hand CERN's writing guidelines suggest that there is an exception: "And note that it is always kelvin, even when plural (not kelvins or degrees kelvin)."
Given all of this, here is my question: Is the SI standard actually to pluralize kelvins, as would be suggested from the quotations from the official SI guides above? Is this officially stated anywhere in some standards organization's guidelines? Or, is there some rationale given somewhere for the continued use of plural "kelvin" (as in the CERN guidelines) or even "Kelvin" (with an apparently anomalous capital)?
Or, is it -- as I suspect -- just a failure to treat the kelvin as an actual SI unit, despite the redefinition from "degrees Kelvin" to "kelvins" that happened decades ago? (Perhaps we just dropped the "degree" but effectively still treat it the same way as Celsius or Fahrenheit?)
-
Originally posted by UltimaOnline:
Flowerd, you're a JC1 student who is trying out JC life without tuition, yes?
So did you understand my replies to your questions? Have they cleared your doubts?
All JC students, feel free (it's literally free) to register an SgForums account to post your H2 / H1 Chem questions in this thread.
Thanks for answering my questions haha! I will ask more questions if I come across anything confusing :)
-
Originally posted by flowerd:
Thanks for answering my questions haha! I will ask more questions if I come across anything confusing :)
No prob :) -
Hi i have a question. For molecules such as 3-methylhexane, which atom/substituent does the dash, wedge and line attach to for 3D structures?
Is there any general rule on how to draw 3D structures or can i attach the atoms to any wedge, dash and line?
-
Another question. Why is the Kc and Kp values only affected by the changes in temperature?
-
Hi ultima,I have a question.
It's an MCQ question.
'The successive ionisation energies,in kJ mol^-1 of an element X are given below.
870 1800 3000 3600 5800 7000 13200
What is X?'
The options includes 8^O and 52^Te and both are in group 6.How is it pt ossible to diffrentiate them using the I.E?
'The elements Rn ,Fr and Ra are consecutive in the periodic table.
What is the order of their first ionisation energies?'
The answer is Fr->Ra->Rn from least to most endorthermic.
The explanation says that Ra is smaller and has 1 more proton than Fr.
Even though Ra have 86 protons and Fr have 87 protons....
-
Originally posted by glitter58:
Hi i have a question. For molecules such as 3-methylhexane, which atom/substituent does the dash, wedge and line attach to for 3D structures?
Is there any general rule on how to draw 3D structures or can i attach the atoms to any wedge, dash and line?
Top group and any immediately adjacent group must be line. Remaining 2 groups can be dash wedge or wedge dash. -
Originally posted by glitter58:
Another question. Why is the Kc and Kp values only affected by the changes in temperature?
That's how humans *define* Kc and Kp, to be affected only by temperature. Position of equilibrium shifting doesn't necessarily mean Kc or Kp changing, that's the case only if temperature changes, in all other changes (ie. moles or molarity or partial pressures of reactants or products), the position of equilibrium shifts because Kc or Kp does *not* change (since temperature didn't change). -
Originally posted by Carychidestar:
Hi ultima,I have a question.
It's an MCQ question.
'The successive ionisation energies,in kJ mol^-1 of an element X are given below.
870 1800 3000 3600 5800 7000 13200
What is X?'
The options includes 8^O and 52^Te and both are in group 6.How is it pt ossible to diffrentiate them using the I.E?
'The elements Rn ,Fr and Ra are consecutive in the periodic table.
What is the order of their first ionisation energies?'
The answer is Fr->Ra->Rn from least to most endorthermic.
The explanation says that Ra is smaller and has 1 more proton than Fr.
Even though Ra have 86 protons and Fr have 87 protons....
After narrowing it down to Group 16, check against the IEs given in the Data Booklet. O doesn't fit the values (note that the 1st few IEs of O are more endothermic than the values given in the MCQ, which clues you in that the element is located below O in the periodic table), hence by elimination (valid approach since this is an MCQ) the correct answer is Te.Fr 1st IE less endothermic than Ra, because both in same period but Ra has 1 more proton. Rn 1st IE most endothermic of the 3 elements because electron is removed from a valence shell that's inner (ie. closer to nucleus) compared to Fr and Ra.
-
Originally posted by UltimaOnline:
Top group and any immediately adjacent group must be line. Remaining 2 groups can be dash wedge or wedge dash.What do you mean by top group and any adjacent group?
-
Originally posted by glitter58:
What do you mean by top group and any adjacent group?
For a tetrahedral molecular geometry, there are 4 groups (bonded to the central atom). One is on top, 2 groups are adjacent (to the top group), and the last group is directly below the top group (relative to the central atom).This is how we draw it on 2D paper. In 3D reality of course, each of the 4 groups are directly adjacent to all 3 other groups.
-
^_^
-
BedokFunland JC Challenge Qn on Gases
Sketch the graphs of PV/RT against P, for both an ideal gas as well as a real gas, on the same axes. For the real gas, explain why there is a negative deviation at low pressure, and why there is a positive deviation at high pressure.
-
BedokFunland JC Challenge Qn on Drawing Mechanisms
Images Source : https://en.wikipedia.org/wiki/Methyl_isocyanate
Easy : Draw the curved-arrow electron-flow mechanism for the reaction :
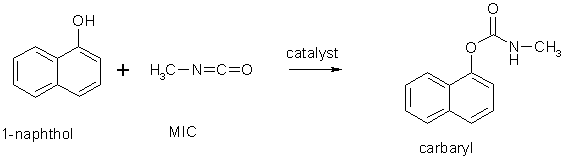
Intermediate : Draw 2 possible curved-arrow electron-flow mechanisms (pericyclic and free radical) for the reaction :

Difficult : Draw the curved-arrow electron-flow mechanism for the extended reaction to generate both products :

(BedokFunland JC students can check their answers with me during tuition, all other students can go check with their school teacher or private tutor.)
-
^_^
-
^_^
-
You do realize, that the Mad Hatter became mad as a result of toxic mercury heavy metal poisoning from his hat?
-
Atrocious karma for atrocious acts. If Cambridge asks, you do know how to explain why and how acid attacks cause tissue damage and disfigurement, don't you?
Man throws acid on ex-lover before killing himself
https://sg.news.yahoo.com/man-throws-acid-ex-lover-killing-himself-235300093.html
Also see :
