2017 H2 Chemistry JC1 & 2 students post your questions here
-
^_^
-
Ascorbic acid (vitamin C), an essential nutrient for humans, is an organic compound containing carbon, hydrogen & oxygen. when 1.000g of ascorbic acid was completely burnt in oxygen, 1.500g of carbon dioxide and 0.405g of water were formed. Calculate the empirical formula of ascorbic acid and determine its molecular formula if its relative molecular mass is 176.
Hi UltimaOnline, how should i start the question? i tried to form the equation of the combustion of a carboxylic acid but i couldn't get the answer :(
Thank you so much! :)
-
Originally posted by keefay:
Ascorbic acid (vitamin C), an essential nutrient for humans, is an organic compound containing carbon, hydrogen & oxygen. when 1.000g of ascorbic acid was completely burnt in oxygen, 1.500g of carbon dioxide and 0.405g of water were formed. Calculate the empirical formula of ascorbic acid and determine its molecular formula if its relative molecular mass is 176.
Hi UltimaOnline, how should i start the question? i tried to form the equation of the combustion of a carboxylic acid but i couldn't get the answer :(
Thank you so much! :)
That's because Vitamin C isn't a carboxylic acid.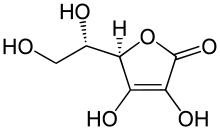
From moles of CO2 generated, find moles of C in sample to be 3.4091x10-2 mol, ie. 4.0909x10-1 g.
From moles of H2O generated, find moles of H in sample to be 4.5x10-2 mol, ie. 4.5 x 10-2 g.
Hence mass of O in sample = 1.00 - (4.0909x10-1 + 4.5x10-2) = 5.4591x10-1 g, ie. 3.4119x10-2 mol.
Moles of Vitamin C = 1.00 / 176 = 5.6818x10-3 mol.
Hence mole ratio of VitaminC to C to H to O is 5.6818x10-3 : 3.4091x10-2 : 4.5x10-2 : 3.4119x10-2, which simplifies to 1 : 6 : 8 : 6.
Hence molecular formula of Vitamin C = C6 H8 O6
No prob, KeeFay! :)
-------------------------------------------------
BedokFunland JC Challenge Qn
Cambridge can ask as an A grade H2 A level exam question, "Identify the most acidic H atom, hence or otherwise, explain why Vitamin C is acidic."
(Interested JC students go ask your school teacher or private tutor for the answer)
-
hi ultimaonline,
for S2O8^2-, the OS of S is +6. i figured it out through drawing the structure but i don't get the same answer using O level method. why is it so?
thank you so much! (:
-
Originally posted by keefay:
hi ultimaonline,
for S2O8^2-, the OS of S is +6. i figured it out through drawing the structure but i don't get the same answer using O level method. why is it so?
thank you so much! (:
Edited to add in italicsBecause the O level method is just an average value and makes simplified assumptions about common OS values for various elements which aren't always correct in context, while my BedokFunland JC formula for OS (which only the best few Singapore JC teachers are familiar with) is superior in that you can use it to calculate the OS of individual atoms in individual resonance contributors.
In more complicated molecules and ions such as the S2O8 2- ion, you *must* use my BedokFunland JC formula to arrive at the correct OS. A recent Singapore H2 A level exam question in which Cambridge asked for the OS of a certain atom, also requires my BedokFunland JC formula to arrive at the correct OS.
BedokFunland JC FTW! ;Þ
-
Hi UltimaOnline,
I just want to say a huge thank you to you! I got my desired grade for chemistry in the A Levels.
Your help was amazing, just like the reaction between sodium and water :D
-
Congrats, glad to hear that, SuperCat! ;)
 Originally posted by supercat:
Originally posted by supercat:Hi UltimaOnline,
I just want to say a huge thank you to you! I got my desired grade for chemistry in the A Levels.
Your help was amazing, just like the reaction between sodium and water :D
-
Hi Ultima.I have a question that I would like you to answer.
"When 10cm cube of a gaseous hydrocarbon were exploded with 100cm cube of oxygen,the residual gas occupied 70 cm cube.After shaking these residual gases with aqueous sodium hydroxide,the final volume was 20 cm cube.All volumes were measured at room temperature and pressure.Calculate the molecular formular of the hydrocarbon."
Does the residual gas includes the excess,unreacted oxygen?(CO2+O2+H2O)
The final residual gas is (O2+H2O)?
Since CO2 reacts with NAOH.
I'm not sure how to solve the 'residual gas' type of combustion analysis.I can solve the normal ones though.
Ans is C5H12
I got 5 for C but I have no idea how to get H...
I just compare the volume ratio and mole ratio
-
Dear UltimaOnline,
I am a J1 this year, and I've just started my first Chem tutorial on Atomic Structure. However I'm stuck on this question. Can you help me with it?
Give a brief explanation of the following observation in terms of ionisation energy.
Orange street-lamps contain sodium with a small amount of neon, and light is produced when gaseous atoms are ionised in an electric field. It is observed that when the street-lamps are turned on, a red glow characteristic of neon is first emitted and the orange glow of sodium predominates after a time.
-
Yo Carychidestar 2017 JC1 student.
70 cm3 = unreacted O2 + CO2 (H2O is liquid at rtp)
Since 20cm3 = unreacted O2, hence 50cm3 = CO2
Since 80 cm3 of O2 used per 10 cm3 of hydrocarbon, hence (5 + y/4) = 8, ie. y = 12
Hence molecular formula of hydrocarbon is C5H12
Originally posted by Carychidestar:Hi Ultima.I have a question that I would like you to answer.
"When 10cm cube of a gaseous hydrocarbon were exploded with 100cm cube of oxygen,the residual gas occupied 70 cm cube.After shaking these residual gases with aqueous sodium hydroxide,the final volume was 20 cm cube.All volumes were measured at room temperature and pressure.Calculate the molecular formular of the hydrocarbon."
Does the residual gas includes the excess,unreacted oxygen?(CO2+O2+H2O)
The final residual gas is (O2+H2O)?
Since CO2 reacts with NAOH.
I'm not sure how to solve the 'residual gas' type of combustion analysis.I can solve the normal ones though.
Ans is C5H12
I got 5 for C but I have no idea how to get H...
I just compare the volume ratio and mole ratio
-
Yo 2017 JC1 student Lockheed2000.
This is an interesting question that was asked by Cambridge in a TYS qn, yet a proper understanding of how the lamp works actually goes beyond the H2 syllabus.
For the simplified version that will suffice for the H2 syllabus (which being oversimplified for A level purposes, is unfortunately inaccurate and misleading in several ways) :
Neon is already gaseous, while sodium is solid, under standard conditions. Hence upon switching on the lamp, the neon gas is ionized first, producing a red glow, while it takes some time for the sodium metal to be vaporized then ionized, then producing the yellow-orange flow of sodium. Na(g) having a less endothermic 1st ionization energy compared to Ne(g), ionizes more readily, and hence being present in a larger quantity, its yellow-orange glow thus predominates.
(Beyond H2 syllabus, for those interested)
But the above simplified A level H2 version is misleading in several ways. Firstly, the ionization itself doesn't produce the glow. The glow occurs when the atoms (whether neon or sodium) transit from a higher energy state, to a lower energy state, in the process emitting electromagnetic radiation or photons of a specific frequency corresponding on that element's emission spectrum (that sodium has a brighter glow than neon, is actually due to their different emission spectrums, rather than the misleading A level explanation given above). This actually happens after, and is separate from, the ionization process.
When the lamp is turned on, the electricity applied ionizes and oxidizes the Ne(g) at the anode, and the Ne+(g) cations are accelerated by the applied electric field toward the cathode, in the process colliding with Ne(g) atoms, which transfer their electrons to the Ne+(g) cations. The Ne(g) atoms (having lost an electron during the collisions), ionize into Ne+(g) and accelerate toward the cathode, while the Ne+(g) cations (having gained an electron during the collisions), become reduced to Ne(g) *and* return to a lower energy state, emitting a photon of light of neon's characteristic frequency (ie. red glow) corresponding to neon's emission spectrum.
The above process generates the heat required to first vaporize the Na(s) to Na(g), and then ionize the Na(g) to Na+(g) at the anode, and thereafter, the same collision process described above with Ne(g) and Ne+(g), then takes place with the Na(g) and Na+(g), resulting in the emitting of photons of light of sodium's characteristic frequency (ie. bright yellow-orange glow) corresponding to sodium's emission spectrum. In practice, inter-elemental collisions between the neon and sodium atoms and ions do occur as well.
Advice to JC students : always first understand the simplified A level concepts first (A levels is a transition bridge between O level child's play, and the real Uni level science), and then only if you're interested and able to handle it, delve into the more correct Uni level details.
Originally posted by Lockheed2000:Dear UltimaOnline,
I am a J1 this year, and I've just started my first Chem tutorial on Atomic Structure. However I'm stuck on this question. Can you help me with it?
Give a brief explanation of the following observation in terms of ionisation energy.
Orange street-lamps contain sodium with a small amount of neon, and light is produced when gaseous atoms are ionised in an electric field. It is observed that when the street-lamps are turned on, a red glow characteristic of neon is first emitted and the orange glow of sodium predominates after a time.
-
Dear UltimaOnline,
my A level result is unlikely to secure a place in local uni and financially I can't go private uni. Neither can I repeat JC2 asI passed all subjects. I have never attended any tuition classes all my life due to financial constraint.
Would like to hear your advice as I will have to retake my A level in 2017.
1. should I register for the new syllabus or stick to the old syllabus last year?
2. will self study using existing notes from JC1/JC2 be sufficient (can't afford private school) ? Or do I need to go for tuition ?
3. If I work part time to pay for tuition fee, do I go for exisiting group lesson or 1 to 1 lesson as I will be a private candidate.
4. how should I get started with my preparation from here? Begin with topical practice or focus on TYS?
Please help and thank you.
-
Hi Alive17,
1. The old 9647 H2 Chem syllabus is available upon request, you have to email SEAB. Whether to register for the new syllabus or old syllabus, depends on whether you intend to go for H2 Chem tuition. If self-study, then old syllabus. If tuition, then new syllabus.
2. Tuition will definitely help (especially if you're registering for the new syllabus). Go for tuition if you can afford it.
3. Group Tuition ($300+) is a lot cheaper than 1 to 1 Tuition ($600+).
4. Whichever approach works best for yourself.
If you're interested in my BedokFunland JC tuition for H2 Chem, you can PM me.
Originally posted by Alive17:Dear UltimaOnline,
my A level result is unlikely to secure a place in local uni and financially I can't go private uni. Neither can I repeat JC2 asI passed all subjects. I have never attended any tuition classes all my life due to financial constraint.
Would like to hear your advice as I will have to retake my A level in 2017.
1. should I register for the new syllabus or stick to the old syllabus last year?
2. will self study using existing notes from JC1/JC2 be sufficient (can't afford private school) ? Or do I need to go for tuition ?
3. If I work part time to pay for tuition fee, do I go for exisiting group lesson or 1 to 1 lesson as I will be a private candidate.
4. how should I get started with my preparation from here? Begin with topical practice or focus on TYS?
Please help and thank you.
-
Hi Ultimaonline,
thanks for your quick response.
If I attend group tuition I have to take the new syllabus which I have no confidence. But sticking to old syllabus I will not be able to find lesson specially for private candidate right?
All my friends are doing fine so I won't have any study buddy to go for the same syllabus. May I know if the new and old syllabus differ greatly?
-
Originally posted by Alive17:
Hi Ultimaonline,
thanks for your quick response.
If I attend group tuition I have to take the new syllabus which I have no confidence. But sticking to old syllabus I will not be able to find lesson specially for private candidate right?
All my friends are doing fine so I won't have any study buddy to go for the same syllabus. May I know if the new and old syllabus differ greatly?
Well if you attend my tuition, you can still stick to the old syllabus if you wish. Coz my BedokFunland JC tuition is a small group tutorial system with personalized attention (so whether you take the old or new syllabus, I can help you no prob), not like the large group mass lecture system of most other H2 Chemistry tuitions.The old syllabus and new syllabus has a number of differences, but the change isn't anything earth-shaking, and it isn't anything you can't cope with, no worries.
-
For the past few decades, medical science has warned that saturated fats cause cardiovascular disease, but little was known or mentioned about trans fats. In recent years however, medical researchers from around the world, including Cambridge University, has found that on the contrary, there was no link between saturated fats and cardiovascular disease, but instead there was a strong link between trans fats and cardiovascular disease. So it turns out saturated fats was the innocent wrongly maligned guy, and trans fats was the evil hidden twin brother carrying out the murders.
BedokFunland JC's A level H2 Chemistry Qns :
What are saturated fats, and why are they called "saturated"? What are trans fats, and why are they called "trans"? Suggest how trans fats could contribute to cardiovascular disease. -
Thanks for the reply.
' 10cm3 of a hydrocarbon Z were exploded with 100cm3 of oxygen in excess.The volume of the gaseous products was 80cm3 but was decreased to 40 cm3 when these products were bubbled through aqueous akali.All volume were measured under the same conditions of temperature and pressure.What is the molecular formula of Z?'
How do I solve problems like this does not give the conditions(temperature and pressure)?
I am not able to determine if steam is also part of the residual gases.
I only know that CO2 is 40cm3.
AND
A completely off-topic question: When do you think the A level assessment books are going to come out?(Updated according to new syllabus)
Due to syllabus changes,the books that are on the shelves of popular bookstores currently aren't really useful.
Just based on your own experience.
Thanks in advance.
-
!!!Originally posted by Carychidestar:
Thanks for the reply.
' 10cm3 of a hydrocarbon Z were exploded with 100cm3 of oxygen in excess.The volume of the gaseous products was 80cm3 but was decreased to 40 cm3 when these products were bubbled through aqueous akali.All volume were measured under the same conditions of temperature and pressure.What is the molecular formula of Z?'
How do I solve problems like this does not give the conditions(temperature and pressure)?
I am not able to determine if steam is also part of the residual gases.
I only know that CO2 is 40cm3.
AND
A completely off-topic question: When do you think the A level assessment books are going to come out?(Updated according to new syllabus)
Due to syllabus changes,the books that are on the shelves of popular bookstores currently aren't really useful.
Just based on your own experience.
Thanks in advance.
You don't need temp and pressure, coz such qns will not require the use of PV=nRT. H2O is always taken to be liquid under standard conditions, unless the question implies that H2O is gaseous under the question's non-standard conditions.Oxygen used = 60 = 10(4+y/4) hence y = 8, ie. hydrocarbon is C4H8
The 2016 TYS is already out, but since the latest 2016 paper is old syllabus, hence you will *not* be able to get any TYS updated to new syllabus before the 2017 A level exam. This has always been the case for every pioneer batch of syllabus change. No worries, doing the old syllabus TYS is still useful, and your school teachers will inform you of the parts of the syllabus that have changes, as well as give you adequate practice for the new content not in the old syllabus exam papers.
-
I love chem
-
I love chem
-
i love chem
-
I love chem
-
^_^
-
I love chem
-
2017 BedokFunland JC H2 Chemistry Challenge Qn
Draw the full curved-arrow electron-flow multi-step reaction mechanism for the hydrolysis followed by dehydration of (i) aluminium chloride and (ii) silicon tetrachloride, including an appropriate representation of the transition from simple molecular reactant, to ionic product (for i) and giant covalent lattice product (for ii).
Also draw the full curved-arrow electron-flow multi-step reaction mechanism for the complete hydrolysis of magnesium nitride to generate a solid and a gas. The correct stoichiometry must be accurately reflected in the reaction mechanism.
Answer :
BedokFunland JC students can ask me during tuition. Other students can go ask your school teacher or private tutor.