2016 H2 Chemistry JC1 & 2 students post your questions here
-
8. (pg 204 reaction II) why do we need to deprotonate NH2OH first? Can reaction be carried out without deprotonation?
9. Is there a mistake in the mechanism in fig 7.9. ? Is the nucleophilic addition - elimination reaction and mechanism in syllabus?
10. (pg 207 tip 4) Is it due to hydride shift?
11. (pg 215) Does all phenyl containing tertiary alcohol form benzoic acid under prolonged heating with acidified KmO4? (pg 214) Do all tertiary alcohol without benzene ring form ketone and carboxylic acid? Do all pri (with K2Cr2O7) and sec alcohol with benzene ring still form aldehyde and ketone when oxidised?
12. (pg 228- 229 example 7.17) For synthesis of 2-methylprop-2-enal, isomer II is used (answer stated isomer III). Why is 2-methylprop-2-enal formed at the last step? It is minor product according to Zaitsev's rule (right?). Or does the presence of C=O result in 2-methylprop-2-enal being the major product?
-
Originally posted by Ephemeral:
My Qs come from George Cheong Organic Chem book:
1. Must a tertiary alcohol be attached to 3 R groups? What if there is a double bond and hence there is only 2 R-groups?
2. Is water slightly acidic at rtp? (Pg 187) Do we just take it as water is neutral at rtp when answering Qs (for acid-base equilibria)?
3. (Pg 190) Why is organic solvent required to dissolve all 3 compounds? Why can't the experiment be carried out just as it is?
4. I don't understand Example 7.7 on pg 191. What do the pKa values mean and how do we use them to solve the Q?
5. Is formation of ester with excess alcohol and conc h2so4 at 140 degree celsius in syllabus?
6. Why is H3PO4 a weaker oxidising agent? What are the relative strength of some of the oxidising agents? (Pg 194)
7. (pg 196 fig.7-5) Why doesn't Et-O-H form protonated ethanol when it abstracts a H atom?
Q1. Then the functional group is called enol, not tertiary or secondary alcohol.Q2. Yes, you can regard tap water to be of neutral pH, unless the question specifies otherwise. For instance, the qn may ask why rain water (even in non-polluted areas) is slightly acidic (you know why?). On pg 187, George Chong is explaining why the Ka of water is 1.8 x 10^-16, why the pKa of water is 15.74, and why the Kw of water is 14.0 ; which has nothing to do with your own question. Does this mean you don't understand George Chong's explanation and the entire page? I've already explained this myself a few years ago on my BedokFunland JC website here : http://www.infinity.usanethosting.com/Tuition/H2Chem_What_is_the_pKa_of_Water.jpg
Q3. The objective of the experiment itself is to use different solvents to separate out the different solutes (ie. via their different solute-solvent affinities). So how can you carry out the experiment (which is about using solvents) if you don't use the solvents? Do you even understand what the experiment is about? I've also already posted on this type of separation techniques using solvents on my BedokFunland JC website here : http://www.infinity.usanethosting.com/Tuition/H2Chem_Separation_of_Organic_Compounds_by_Acid_Base_Solvent_Extractions.jpg
Q4. This qn is from Singapore-Cambridge A levels 2012 P3 Q5. The intramolecular hydrogen bonding in the uninegative conjugate base for the cis isomer, results in the Ka1 for the cis isomer to be larger than the Ka1 for the trans isomer (since the more stable the conjugate base, the stronger the acid), and also results in the Ka2 for the cis isomer to be smaller than the Ka2 for the trans isomer (since for the cis isomer, to dissociate the 2nd proton, you need to endothermically break or dissociate the intramolecular H bond *in addition* to the O-H covalent bond).
Q5. Yes it is. Students aiming for A grade should also be familiar with the electron flow mechanism for both the forward (ie. esterification & nucleophilic acyl substitution & condensation & addition-elimination reaction) and backward (acidic and alkaline hydrolyses) reactions. And if you meant "ether" instead of "ester", Cambridge can also ask you to draw the mechanism to generate the ether, as a challenging A grade exam qn.
Q6. Compared to H2SO4, H3PO4 is a weaker oxidizing agent for 2 reasons : S is more electronegative than P, and the OS of the heteroatom is +6 (in H2SO4) vs +5 (in H3PO4). Using the Data Booklet redox potentials (topic : electrochemistry), you can deduce the relative oxidizing and reducing strengths. For species not included in the Data Booklet, you'll have to apply your knowledge of H2 Chem (across topics) to make reasonable deductions.
Q7. It does, of course. Protonated ethanol CH3CH2OH2+ is an electrophilic intermediate (remember that an atom with a positive formal charge is more strongly electron-withdrawing by induction compared to if it had no formal charge), attacked by a 2nd ethanol molecule, the nucleophile. As this is a primary alcohol, SN2 occurs instead of SN1 (George Chong missed out the curved arrow depicting the elimination of the H2O+ leaving group, draw it into your book yourself).
-
Originally posted by Ephemeral:
8. (pg 204 reaction II) why do we need to deprotonate NH2OH first? Can reaction be carried out without deprotonation?
9. Is there a mistake in the mechanism in fig 7.9. ? Is the nucleophilic addition - elimination reaction and mechanism in syllabus?
10. (pg 207 tip 4) Is it due to hydride shift?
11. (pg 215) Does all phenyl containing tertiary alcohol form benzoic acid under prolonged heating with acidified KmO4? (pg 214) Do all tertiary alcohol without benzene ring form ketone and carboxylic acid? Do all pri (with K2Cr2O7) and sec alcohol with benzene ring still form aldehyde and ketone when oxidised?
12. (pg 228- 229 example 7.17) For synthesis of 2-methylprop-2-enal, isomer II is used (answer stated isomer III). Why is 2-methylprop-2-enal formed at the last step? It is minor product according to Zaitsev's rule (right?). Or does the presence of C=O result in 2-methylprop-2-enal being the major product?
Q8. No, deprotonation is necessary for this reaction because hydroxylamine (and all amines) are too weak a nucleophile to attack the ester (which is a weak electrophile because the partial positive charge on the C atom is only due to induction, not resonance ; resonance is the same reason why the weak nucleophile 2,4-DNPH only reacts with the more electrophilic aldehyde and ketone, but not the less electrophilic carboxylic acids, esters and amides).Q9. Yes, there's an error in the mechanism. While it's not required for the basic H2 syllabus, but if you're serious about getting your A grade, it'll be good for you to understand and be familiar with such mechanisms (not blindly memorize, of course).
For A grade H2 Chem, H3 Chem and Olympiad Chem students, here is the SOCl2 mechanism by James Ashenhurst : http://www.masterorganicchemistry.com/2011/12/03/reagent-friday-thionyl-chloride-socl2
For A grade H2 Chem, H3 Chem and Olympiad Chem students who are seriously intending to study Chemistry, Chemical Engineering, Biochemistry, Pharmacy, Dentistry or Medicine in the University, here is a more advanced discussion on the stereochemistry of the SOCl2 mechanism :
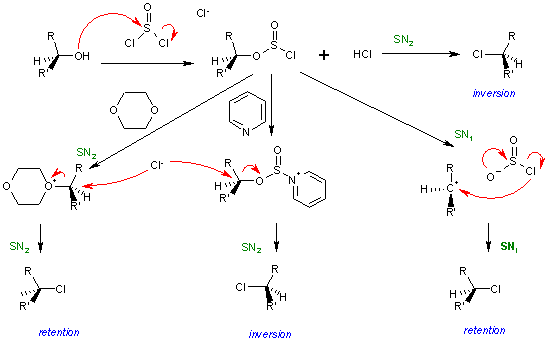
Image source : https://en.wikipedia.org/wiki/SNi
If you have difficulty understanding the diagram above, James Ashenhurst explains the reaction in detail here : http://www.masterorganicchemistry.com/2014/02/10/socl2-and-the-sni-mechanism/Q10. Yes, carbocation rearrangements can be due to hydride or alkyl shifts. See Leah Fisch's YouTube video here : https://www.youtube.com/watch?v=cSW9LDxtuoA, or Khan Academy's YouTube video here : https://www.khanacademy.org/science/organic-chemistry/substitution-elimination-reactions/e1-e2-tutorial/v/carbocations-and-rearrangements
Q11. Yes, and yes. The condition for side-chain oxidation by KMnO4 is that at least 1 benzylic H atom or benzylic OH group must be present.
Q12. Eh look carefully lah, there's only 1 possible dehydration (ie. elimination of H2O) product, as the 2 terminal or beta methyl groups are equivalent.
Also, when it comes to Markovnikov's or Zaitsev's rules, don't blindly apply or quote them. If Cambridge asks why such-&-such is the major product, you'll need to explain *why*, ie. the basis for *how* and *why* Markovnikov or Zaitsev's rule works (or in more challenging qns, may fail to work).
Good that you can suggest suspecting the C=O group, but such groups undermine Markovnikov's rule (see my BedokFunland JC qn : http://www.infinity.usanethosting.com/Tuition/H2Chem_Does_Markovnikov_Rule_Always_Work.jpg), not Zaitsev's rule, so it's irrelevant to this particular question. For an example of how the major product can controlled to be the Hofmann product instead of the Zaitsev product, see https://en.wikipedia.org/wiki/Zaitsev%27s_rule#Steric_effects.
-
for my Q12, I was wondering why the dehydration cannot occur with the H from the aldehyde (taking H from a C that is 'less rich' in H).
Why is the reactivity of halogenating agents as such: F>Cl>Br>I ?
-
Originally posted by Ephemeral:
for my Q12, I was wondering why the dehydration cannot occur with the H from the aldehyde (taking H from a C that is 'less rich' in H).
Why is the reactivity of halogenating agents as such: F>Cl>Br>I ?
I'll deal with your halogenating qn 1st :Your qn is ambiguous, do you refer to halogenation via free radical mechanism, or via nucleophilic (aliphatic or acyl) substitution, or via electrophilic aromatic substitution, etc? Never be ambiguous in your A level exam answers, if Cambridge asks "what is this reaction?" you should specify "halogenation via _________ (mechanism)".
So what exactly are you asking? Provide a context if relevant, eg. a specific TYS or prelim paper qn, or a website link, or a page number from a CS Toh book, or George Chong book, or Chan Kim Seng book, etc.
--------------------------------------------------------
Edited : "for my Q12, I was wondering why the dehydration cannot occur with the H from the aldehyde (taking H from a C that is 'less rich' in H)."
Eh sial lah! From your statement above, it shows you're just blindly memorizing and applying Markovnikov's and/or Zaitsev's rules without a proper understanding of *how* or *why* they usually work (and thus when they sometimes won't work)! You (ie. most Singapore JC students) jialat liao lah, this is the kind of teaching in Singapore JCs which illustrates the superiority of my BedokFunland JC pedagogy : *Understanding* then applying (as opposed to "blindly memorizing then applying" as taught in Singapore JCs).
As to your Zaitsev qn : Ahhh ok, what you mean is you're thinking of generating the ketene functional group from the acyloin functional group, well you could have just said so ;Þ
There are a couple of problems with your proposed reaction. First of all, it's not even thermodynamically feasible to generate a ketene (which is highly unstable and reactive) from an alpha-haloaldehyde/ketone, much less from an alpha-hydroxyaldehyde/ketone (ie. the acyloin functional group).
The dehydration of alcohols to alkene involves the the E2 mechanism (pri & sec alcohols) or E1 mechanism (sec & tert alcohols) catalyzed by an acid (see Jim Clark and Khan Academy and James Ashenhurst).
But when you have an acyloin functional group, it's more kinetically and thermodynamically feasible to protonate the carbonyl group rather than the hydroxy group, in which case hydrolysis of the aldehyde into the germinal diol occurs instead, which is unstable and simply dehydrates back into the aldehyde.
But since it's not entirely impossible or inconceivable (just less kinetically and thermodynamically favourable) for your proposed mechanism pathway to occur (ie. to generate the ketene functional group from the acyloin functional group) as a minor pathway or product via the E2 mechanism (E1 is even less feasible because of the destabilizing inter-nuclei repulsions between the partial and formal positively charged adjacent C atoms in the hypothetical carbocation intermediate), what happens then?
Under the reaction conditions to dehydrate an alcohol into an alkene (ie. concentrated H2SO4 acid catalyst), the highly unstable and reactive ketene product generated would be immediately acid-hydrolyzed into a carboxylic acid (try drawing out the mechanism for this reaction ; Cambridge asked data-based questions about the reaction of ketenes in both the UK / CIE and Singapore A level papers in recent years).
So the bottomline : good that you've considered the possibility* of obtaining a ketene as an alternative product, but unless the question gives you hints that the product is a ketene, you should stick to the more syllabus-familiar product of a normal alpha-carbonylalkene, rather than the more unstable ketene (H2 Chem students are expected to be able to deduce its instability by its structure). But nonetheless (as I said, good that you've thought-out-of-the-box* and considered the ketene product), in the A level exams, if you're not sure if the unusual product (here, the ketene) is a viable alternative product (or even one required by the Cambridge qn), then the exam-smart BedokFunland JC thing to do, is to give both answers (ie. draw structures of both alternative products), with labels or annotations as to which is likely to be the more stable (ie. thermodynamic) product, and hence major product.
* updated : actually it turned out you were just blindly applying Markovnikov's / Zaitsev's rule, not creatively thinking out-of-the-box... *sigh*
-
 Question 3 part a
Question 3 part a -
Originally posted by Shinyphua:
 Question 3 part a
Question 3 part a
Eh ur FB private izzit? Can't see the pic lah. Try using Twitter or smth else. -
When you treat nitrobenzene with Sn in conc HCl to form phenylamine, why do you still need to add NaOH after that?
-
Originally posted by Ng.keebin:
When you treat nitrobenzene with Sn in conc HCl to form phenylamine, why do you still need to add NaOH after that?
Because in the presence of acidic conc HCl, the product is the protonated conjugate acid phenylammonium ion (which is water soluble due to ion - permanent dipole interactions and hence difficult to separate from the other species present), which must thus be deprotonated by excess NaOH(aq) to obtain the desired product phenylamine, which must then be extracted via steam distillation.The experimental details of steam distillation are beyond A levels, but Cambridge can (and have in past A level papers) set challenging questions on the chemistry concepts underlying practical procedures (including steam distillation). Students seriously intending to score a distinction A grade for your A levels, are strongly advised to study the following webpages.
---------------------------------------------------------------------------------
Source : http://www.chemguide.co.uk/organicprops/aniline/preparation.html
Sodium hydroxide solution is added to the product of the first stage of the reaction. The phenylamine is formed together with a mixture of tetrahydroxozincate(II) and hexahydroxozincate(IV) complex tin ions formed from the tetrachlorozincate(II) and hexachlorozincate(IV) complex tin ions present during the acidic HCl stage. The phenylamine is finally separated from this mixture. The separation is long, tedious and potentially dangerous (and thus not experimentally carried out at A levels, but questions can still be asked on the procedure), involving steam distillation, solvent extraction and a final distillation.
---------------------------------------------------------------------------------
Source : http://chemistry.stackexchange.com/questions/10531/preparation-of-phenylamine-aniline
This is the procedure I (a Chemistry undergraduate student) carried out in the lab :
1.Reduction of nitrobenzene by using tin and HCl acid
2.After the completion of the reaction, NaOH added to neutralize excess acid and dissolve any Sn(OH)2(s)
3.Steam distillation is carried out to obtain suspension of phenylamine
4.Salting carried out where NaCl salt is added (called 'salting'). Aqueous layer discarded
5.Solvent extraction : dry ether added and mixture shaken vigorously. Aqueous layer discarded
6.Distillation under reduced pressure (ie. partial vacuum) carried out and solid phenylamine left in flask
My questions:
1. Instead of carrying out distillation under reduced pressure at the end of the procedure to remove the ether, could I have just carried out simple distillation?
2. Why is steam distillation suitable in part 3? Could I have used vacuum distillation here?
(See webpage for the answers to this Chem undergrad's qns).---------------------------------------------------------------------------------
Source : http://rod.beavon.org.uk/phenylamine_prep.htm
•What is the reaction between the tin and hydrochloric acid?
•Sn + 2HCl → Sn2+ + 2Cl- + H2
•What is the reducing agent, and to what is it oxidised?
•The reducing agent is Sn2+, which is oxidised to Sn4+.
•What is the initial precipitate, and what does it become on addition of excess sodium hydroxide?
•Tin(IV) hydroxide Sn(OH)4 (s). This is amphoteric, and with more alkali forms the soluble Sn(OH)6 2- hexahydroxozincate(IV) complex ion.
•Why is more water added to the solution?
•This enables a technique called steam distillation. In the large-scale preparation steam is blown through the mixture; in this one the steam is generated in situ. Phenylamine distils over with the steam; any unchanged nitrobenzene and the inorganic materials do not.
•Why is the initial distillate cloudy?
•It is a mixture of water and phenylamine which formas a cloudy emulsion
•What is the clear condensate?
•Water; by now all the phenylamine has distilled over.
•What is the purpose of adding sodium chloride?
•Phenylamine is significantly soluble in water, but very much less so in saturated sodium chloride solution. This process is called 'salting out'.
•What is the purpose of adding ethoxyethane?
•Solvent extraction; phenylamine is much more soluble in ethoxyethane than it is in water.
•Why are two portions of 4 cm3 used, rather than a single 8cm3 portion of of ethoxyethane?
•The theory of solvent extraction is a branch of chemical equilibria. It can be mathemically shown (and can be asked in data-based A level exam questions) that any solvent extraction is more effective if a given volume of extracting solvent is used in several portions rather than in a single one.
•Suggest a reason for using KOH as a drying agent, rather than the more conventional calcium chloride or sodium sulphate.
•The use of potassium hydroxide would also eliminate any traces of hydrochloric acid in the phenylamine.
•Why must all flames in the laboratory be extinguished?
•The vapour of ethoxyethane is very dense and will creep along bench-tops over a considerable distance. It is possible for an explosion to ensue even if the source of ignition is several metres away from where the ethoxyethane is being used.
•Why is the water run out of the condenser?
•Phenylamine has a sufficiently high boiling temperature for an air condenser to be efficient at condensing the vapour.
•Why is the phenylamine kept condensing on the thermometer bulb before allowing distillation to proceed?
•This allows the thermometer to come into thermal equilibrium with the vapour - a general necessity in distillation, but needing more care than usual if the boiling temperature of the distillate is high. Phenylamine boils at 184oC. -
https://twitter.com/_hykelchem/status/707837536685588480?s=09
I don't really know how to answer both question 7 and 8. Please help!
-
Originally posted by hykel:
https://twitter.com/_hykelchem/status/707837536685588480?s=09
I don't really know how to answer both question 7 and 8. Please help!
For the HCJC qn, only C is possible, since the halogen radical only abstracts H atoms in propagation steps, leaving behind alkyl radicals which can form new C-C bonds with other alkyl radicals in termination steps. Options A, B and D require cleavage of C-C bonds, and/or other alkane molecules such as methane and ethane to be available. Try drawing out the reaction mechanisms, you'll find only C is possible.For the MJC qn, the 2 factors which determine product distribution during free radical substitution are : no. of H atoms substitutable, and stability of alkyl radical intermediates (aka reactivities of pri vs sec vs tert H atoms). Based on no. of H atoms substitutable, ratio of 1-bromo-2-methylpropane to 2-bromo-2-methylpropane should be 9 : 1. Based on stability of alkyl radical intermediates, ratio of 1-bromo-2-methylpropane to 2-bromo-2-methylpropane should be 1 : 6. Hence, actual ratio obtained (ie. combining the 2 factors mathematically by multiplication) = 9 x 1 : 1 x 6 = 9 : 6 = 3 : 2
-
When you treat nitrobenzene with Sn in conc HCl to form phenylamine, why do you still need to add NaOH after that?
After the addition of Sn in conc HCl, it will form C6H5NH3+, as phenylamine being a base, will react with conc HCl to form a salt. Thus, the addition of NaOH is to turn the salt C6H5NH3+ back into phenylamine.
-Quintessential Education
 Find out more: http://qeducation.sg/
Find out more: http://qeducation.sg/ -
https://twitter.com/_hykelchem/status/710759882488549376?s=09
I'm not sure how to get compound A and B. Please help :)
-
Originally posted by hykel:
https://twitter.com/_hykelchem/status/710759882488549376?s=09
I'm not sure how to get compound A and B. Please help :)
Step 3 is nucleophilic acyl substitution (addition-elimination) to get amide group. Step 4 is nucleophilic aliphatic substitution (SN2) to get ether group.In step 6, thionyl chloride converts carboxylic acid group to acyl chloride group.
For A grade H2 Chem, H3 Chem and Olympiad Chem students, here is the SOCl2 mechanism by James Ashenhurst : http://www.masterorganicchemistry.com/2011/12/03/reagent-friday-thionyl-chloride-socl2
For A grade H2 Chem, H3 Chem and Olympiad Chem students who are seriously intending to study Chemistry, Chemical Engineering, Biochemistry, Pharmacy, Dentistry or Medicine in the University, here is a more advanced discussion on the stereochemistry of the SOCl2 mechanism :

Image source : https://en.wikipedia.org/wiki/SNi
If you have difficulty understanding the diagram above, James Ashenhurst explains the reaction in detail here : http://www.masterorganicchemistry.com/2014/02/10/socl2-and-the-sni-mechanism/ -
Hello! Keebin and I did the organic chem deductive elucidation killer Q13 and we got a few answers as attached -
When O C4H6O6 is heated with concentrated sulfuric(VI) acid, two possible products M and G with molecular formulae C4H2O4 and C8H8O10 are formed as a result of intramolecular and intermolecular esterifications, respectively. Elucidate O, M, G.https://twitter.com/123chem2016/status/711185220322463745
M4 is obtained frm O1 as well. Are they all possible? As for the G compounds, degree of unsaturation for G3 is 7 instead of 5. Technically G1 can also under elimination (of water) to form another C=C...
Benzaldehyde is less reactive than methanal. Why is that so? Why does delocalisation of electrons through resonance reduce the electrophilicity of carbonyl carbon and make it less reactive? If the the electrons are delocalised over to the O atom, then doesn't it increase the partial positive charge on carbonyl C and hence make it more susceptible to nucleophilic addition?
When Grignard reagents react with carbonyl, a covalent bond is formed between O and MgX. Why can't it be an ionic bond?
-
Originally posted by Ephemeral:
Hello! Keebin and I did the organic chem deductive elucidation killer Q13 and we got a few answers as attached -
When O C4H6O6 is heated with concentrated sulfuric(VI) acid, two possible products M and G with molecular formulae C4H2O4 and C8H8O10 are formed as a result of intramolecular and intermolecular esterifications, respectively. Elucidate O, M, G.
https://twitter.com/123chem2016/status/711185220322463745
M4 is obtained frm O1 as well. Are they all possible? As for the G compounds, degree of unsaturation for G3 is 7 instead of 5. Technically G1 can also under elimination (of water) to form another C=C...
Benzaldehyde is less reactive than methanal. Why is that so? Why does delocalisation of electrons through resonance reduce the electrophilicity of carbonyl carbon and make it less reactive? If the the electrons are delocalised over to the O atom, then doesn't it increase the partial positive charge on carbonyl C and hence make it more susceptible to nucleophilic addition?
When Grignard reagents react with carbonyl, a covalent bond is formed between O and MgX. Why can't it be an ionic bond?
All your structures are wrong. The question specified the products are generated as a result of intra and inter molecular esterifications, and dehydration of alcohol to alkene is not involved (as verified by the degree of unsaturation). O1 (hence M1 and G1 as well) is/are wrong because geminal diols spontaneously dehydrate to generate aldehydes / ketones, with a negligible molarity of the geminal diol present at equilibrium (it's true that KMnO4 and K2Cr2O7 oxidizes aldehydes via their hydrolysis into geminal diols, but KMnO4 and K2Cr2O7 are able to thusly shift the position of equilibrium to the RHS as the geminal diol is oxidized into carboxylic acid). Your O2 (and thus M2, G2, M3, G3 as well) is/are wrong because your M2, M3 and G3 suffers from excessive ring strain due to angle strain (an approximately 90 deg 4-membered ring is already strained from sp3 C atoms ideally 109.5 deg, let alone sp2 vinylic C atoms ideally 120 deg C). Now that I've revealed your structures of O are wrong, there's only 1 possible structure left, which is the correct structure of O, and hence the correct M and G follows accordingly.Because of both sterics and electronics : the electrophilic C atom in benzaldehyde suffers from greater steric hindrance (for the incoming nucleophile) compared to methanol ; in terms of electronics methanal has a valid resonance contributor in which the carbonyl C atom has a unipositive formal charge, while in benzaldehyde the unipositive formal charge is delocalized from the benzylic C atom over to the ortho, para and ortho C atoms in the benzene ring, consequently in their resonance hybrids, the magnitude of partial positive charge and hence electrophilicity, is significantly greater in the methanal C atom than in the benzaldehyde C atom.
The O-Mg bond in the intermediate R-O-Mg-X upon nucleophilic addition of the Grignard reagent, has significant covalent and ionic character both. Unless otherwise specified by the question, Cambridge will accept it whether you draw & describe the bond as ionic (with significant covalent character), or as covalent (with significant ionic character). It's actually better to draw it as ionic (ie. O- +Mg with formal charges between them), to show Cambridge that you're aware of the greater magnitude of electronegativity difference between O & Mg compared to C & Mg (which is more covalent than ionic).
-
Hello! I love Chemistry.
-
A couple of JC students asked in the "Lurkers" H2 Chemistry thread a CS Toh question based on the hydrolysis of a germanium chloride. For the fun of it, I then decided to write a 2016 BedokFunland JC H2 Chemistry question based on the hydrolyses of germanium chlorides, for your entertainment and pleasure. If you can correctly answer my BedokFunland JC questions, it's indicative of getting an A grade for your Singapore-Cambridge H2 Chemistry A level exams. After all, I always get the distinction A grade myself every year, so I know exactly what it takes.

-
H2 Chemistry students are often confused between Iodometry and Iodimetry, the 2 commonly encountered types of redox titrations involving iodine. If you're uncertain of the difference, go check it up yourself now.
Tis the Age of the Internet,
Google by thy Sword,
Wikipedia be thy Shield- BedokFunland JC
-
https://twitter.com/itsliyingg/status/715502704089403392
Qns 10 parts d and e
-
Originally posted by Liying98:
https://twitter.com/itsliyingg/status/715502704089403392
Qns 10 parts d and e
Compared to Singapore JC questions, Cambridge will be more specific and less ambiguous on exactly what they want you to comment on. Don't worry too much about silly Singapore JC questions in which different school teachers (even within the same JC, let alone across different JCs) routinely disagree with each other.Formation enthalpy of Al2O3 is significantly (almost 3 times) more exothermic than formation enthalpy of MgO, despite the more endothermic 1st + 2nd + 3rd ionization enthalpies for Al compared to Mg, illustrating both the stronger ionic (with significant covalent character) bonds for Al2O3 compared to MgO (due to Al3+'s higher cationic charge density compared to Mg2+), as well as the greater thermodynamic and thermal stabilities of Al2O3 over MgO.
Al2O3 is expected to be more stable (ie. both lattice formation enthalpy and formation enthalpy more exothermic) compared to Al2S3, due to the higher anionic charge density of O2- compared to S2-.
-
what's the difference betwen lattice formation enthalpy and formation enthalpy?
-
Originally posted by Flying grenade:
what's the difference betwen lattice formation enthalpy and formation enthalpy?
Former is from gaseous ions to solid lattice, under standard conditions. Latter is from elements to compound, under standard conditions. -
What is a simple chemical test to differentiate between 2,4,6-tribromophenol and 2,4,6-trichlorophenylamine? (Cannot use FeCl3)
Can aq NaOH or limited RX in ethanol be used?
-
Originally posted by Ephemeral:
What is a simple chemical test to differentiate between 2,4,6-tribromophenol and 2,4,6-trichlorophenylamine? (Cannot use FeCl3)
Can aq NaOH or limited RX in ethanol be used?
What would be your expected observations in your proposed answer? Then you'll know if your method works.And instead of me spoonfeeding you guys all the answers, how about you 100+ lurkers post your own attempted answer? Ideally, let this forum be a discussion amongst you students helping each other out as well, and I'll come in only to guide and advise.