[H2 Chemistry] - Misc TYS qns
-
NJC_2013_P2
1 (b)(ii) For the dilution process can we use distilled water instead? I don't really understand how the dilution process given in the ans scheme works
-
Originally posted by BCME:
TJC_2013_P2
Q1 (iii) How do we know what chemical reagents A and B are?
For B can we use LiALH4? Or must it be something that can react with h20?
Thanks!
Based on description given by the question, you have to propose the identities of A and B. As seen in the mark scheme, there are several acceptable answers for them, as long as it's reasonable.
LiAlH4 can indeed remove water, but it's a lot more expensive and hazardous to use (think explosive H2 gas generated) compared to the required mark scheme answer, which uses cheaper and less hazardous desiccating agents such as calcium salts. As such, Cambridge will not accept LiAlH4 as an answer. -
Originally posted by BCME:
NJC_2013_P2
1 (b)(ii) For the dilution process can we use distilled water instead? I don't really understand how the dilution process given in the ans scheme works
Without dilution, the volume used in the titration is too small and will result in high experimental error. The mark scheme used deionized water, which for A level practical purposes in most cases, is equivalent to distilled water. Of course, because the deionization process only removes ions, not neutral molecules, distilled water is still superior in terms of purity. -
NYJC_P2_2013
4 (b)(iii) Why will the product not be optically active? Even though there is an equal chance of being attacked on either side of the plane i thought that as long as the substituients are different the compoud will be optically active?
-
Originally posted by BCME:
NYJC_P2_2013
4 (b)(iii) Why will the product not be optically active? Even though there is an equal chance of being attacked on either side of the plane i thought that as long as the substituients are different the compoud will be optically active?
You'll get a racemic mixture (ie. equimolar mixture of both enantiomers) and thus the *mixture* (of both optically active molecules) is overall optically inactive. -
Hi UltimaOnline,
A Level 13/P2/Q5b(i)By convention when we write primary structure of the protein we do so from the N to the C terminus. Would it be wrong if I wrote the sequence from the C to the N terminus instead? Also, in this case, where is the C terminus?Thank you! -
Originally posted by gohby:
Hi UltimaOnline,
A Level 13/P2/Q5b(i)By convention when we write primary structure of the protein we do so from the N to the C terminus. Would it be wrong if I wrote the sequence from the C to the N terminus instead? Also, in this case, where is the C terminus?Thank you!
For oxytocin, there is no C terminus, because the C terminus (belonging to glycine) has reacted (in a nucleophilic acyl substitution, addition-elimination, condensation reaction) with NH3 to become the amide group you see at the bottom-left corner of oxytocin. You *must* write it the correct way around to get the 1 mark.
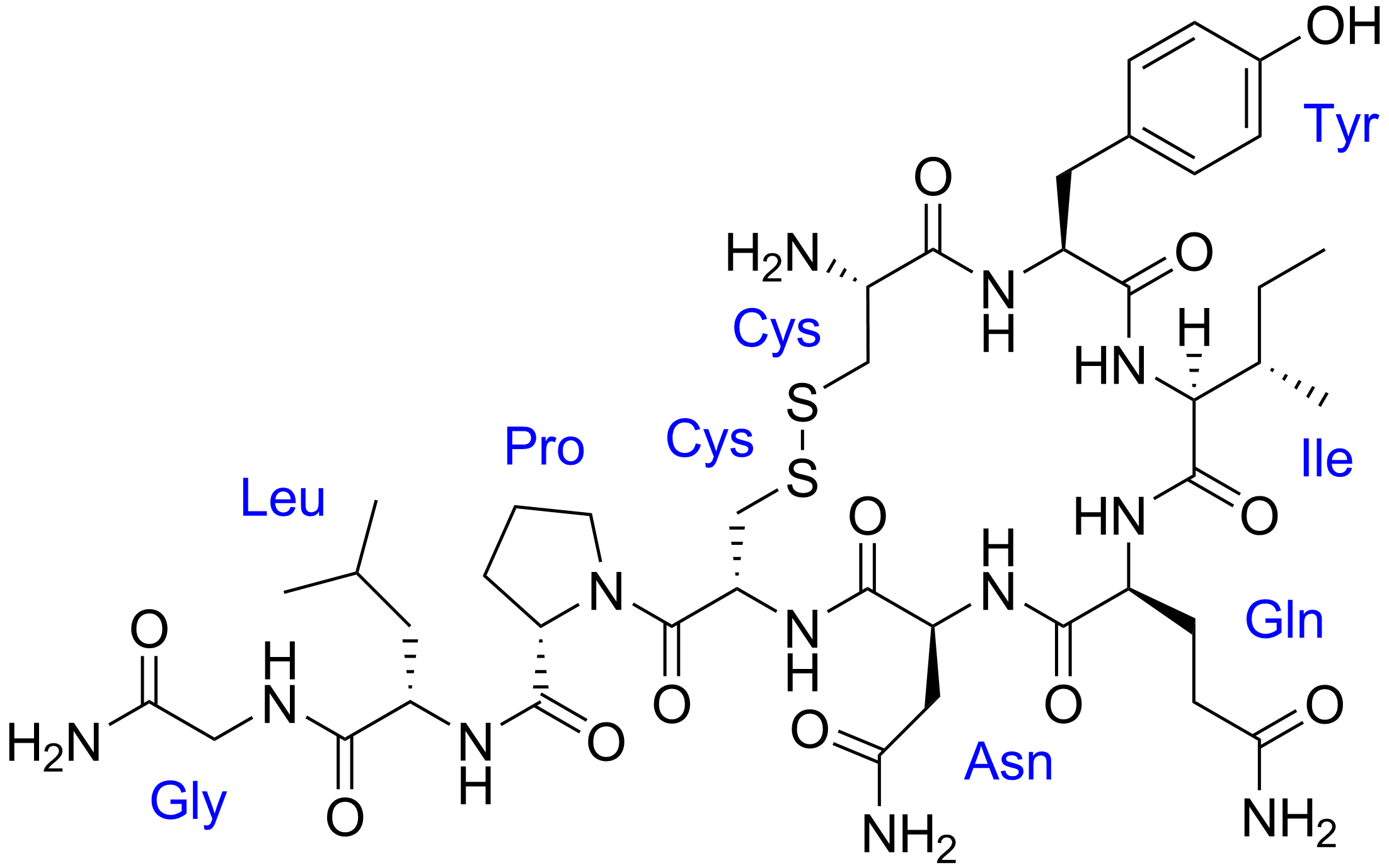
https://en.wikipedia.org/wiki/Oxytocin#Structure -
TYS 2011 Paper 2 question 4e
how do you determine which half equation to use for the V3+ "in a solution containing V3+" like what hints tell u that you are supposed to consider the VO2+ half equations and that there is further reaction?
-
TYS 2011 Qn 4c(ii) can the explanation be that CCl4 is non polar hence unable to form intermolecular bonds with water molecules that are strong enough to overcome the hydrogen bonding In water hence CCl4 is inert?
-
How do gaseous molecules Kp and Kc value relate to each other? Why when u use Kc the value is different if u calculate the gas in terms of partial pressure instead of concentration?
-
Originally posted by BCML:
TYS 2011 Paper 2 question 4e
how do you determine which half equation to use for the V3+ "in a solution containing V3+" like what hints tell u that you are supposed to consider the VO2+ half equations and that there is further reaction?
First, you should be aware (if you didn't know, now you know liao) that the OS of V in the common V containing ions are 0, +2, +3, +4, +5 and +5, in V, V 2+, V 3+, VO 2+, VO2 + and VO3 - respectively.
(Special Note : The equilibrium between the dioxovanadium(V) ion and vanadate(V) ion isn't a redox reaction, but an acid-base equilibrium, similar to the equilibrium between chromate(VI) and dichromate(VI) ; you can figure out exactly which species with this OS is present under acidic vs alkaline conditions, by looking at whether H+ is present on the LHS or RHS).
Observe PbCl4 : obviously the strongest redox effect of PbCl4, is its oxidizing power (since Pb4+ is unstable and wants to be reduced to Pb2+, which is more stable due to lower anionic charge density and/or the inert pair effect).
Hence, Let Pb4+ be reduced to Pb2+, and let V be oxidized from V3+ to VO 2+ (ie. OS change from +3 to +4). Prove using Cell potential = Reduction potential @ Cathode + Oxidation potential @ Anode, that this redox reaction is thermodynamically feasible.
Next, notice that VO +2 (OS of +4) *might* be able to be further oxidized to VO2 + (OS of +5) under acidic conditions. Again prove using Cell potential = Reduction potential @ Cathode + Oxidation potential @ Anode, that this redox reaction is thermodynamically feasible.
Therefore, the observable color change is from green V3+ to blue VO 2+ to yellow VO2 +, using Pb4+ reduced to Pb2+ as the oxidizing agent in both (thermodynamically feasible) redox reactions. Warning : between blue VO 2+ to yellow VO2 +, a green colour can be observed. This is not due to an intermediate OS between +4 and +5 (which is impossible), but it's due to a mixture of blue VO 2+ and yellow VO2 +, which we humans perceive as green (ie. blue + yellow = green), a common exam trick question. -
Originally posted by BCML:
TYS 2011 Qn 4c(ii) can the explanation be that CCl4 is non polar hence unable to form intermolecular bonds with water molecules that are strong enough to overcome the hydrogen bonding In water hence CCl4 is inert?
Totally wrong. Zero marks. This is basic H2 syllabus stuff that you need to memorize (with understanding of course), how can you not know this when Paper 2 is just a few hours away?!? Be careful not to lose easy marks like this, whether in P2 or P3!
Chan Kim Seng + Jeanne Tan : Physical / Inorganic book page 376.
George Chong : Inorganic book page 59.
Jim Clark : simpler A level explanation : http://www.chemguide.co.uk/inorganic/group4/chlorides.html
Rod Beavon : more advanced A level / University level explanation : http://rod.beavon.org.uk/hydrolys.htm
-
Originally posted by BCML:
How do gaseous molecules Kp and Kc value relate to each other? Why when u use Kc the value is different if u calculate the gas in terms of partial pressure instead of concentration?
There is a formula to intercovert Kc and Kp values (which are different because Kc is in molarities, Kp is in partial pressures). This formula isn't required in the H2 syllabus, so Cambridge will provide you with the formula if the question requires you to use it. Or (less likely) if the Cambridge wants to be sadistic, the queston may ask you to derive the formula by yourself (linking H2 syllabus topics Ideal Gas Law PV=nRT with Equilibria Kc & Kp).
Read the following to understand (memorize the final formula if you like, it's simple enough, but don't memorize the derivation process, just understand it can liao).
http://bilbo.chm.uri.edu/CHM112/lectures/KpKc.htm
http://chem-guide.blogspot.sg/2010/04/relation-between-kp-and-kc-derivation.html
http://www.thebigger.com/chemistry/free-energy-and-chemical-equilibria/derive-the-relationship-between-kp-and-kc/
https://www.youtube.com/watch?v=L_PebPmCNuY -
Logging off for tonight liao. Cya guys tomorrow here in this forum after P2 to discuss the P2 questions! Have fun! ;D
-
In Alevel_2011_P3
Q1(e) When amides undergo hydrolysis
RCONH2 + OH- ---> ROO- +RNH2
Why is it not the case for the last reaction? Also what is the role of Br2 in the last reaction?
Thanks
-
Originally posted by BCME:
In Alevel_2011_P3
Q1(e) When amides undergo hydrolysis
RCONH2 + OH- ---> ROO- +RNH2
Why is it not the case for the last reaction? Also what is the role of Br2 in the last reaction?
Thanks
Because of a Uni level reaction mechanism known as the Hofmann rearrangement (which is of course beyond the H2 syllabus, but since part d of the Qn was talking all about it and giving you the info you need, you should therefore suspect part e of the Qn might be linked to part d) :
https://en.wikipedia.org/wiki/Hofmann_rearrangement -
With reference to A-level_2010_P1, Q40.
What makes the COCHCl2 group not be able to participate in an iodoform reaction with alkaline iodine? Is it due to the amide group? So all the amide group C=O are not considered is it?
-
Originally posted by BCME:
With reference to A-level_2010_P1, Q40.
What makes the COCHCl2 group not be able to participate in an iodoform reaction with alkaline iodine? Is it due to the amide group? So all the amide group C=O are not considered is it?
-_-"
Only now (after P2 & P3 over liao, only left P1) then you know this ah? Carboxylic acids, esters & amides do *not* undergo the iodoform reaction, only ethanal (among the aldehydes), alcohols with CH3-CH(OH)-R and ketones with CH3-CO-R undergo the iodoform reaction.