[A level Chemstry] - BuckyBalls
-
Q. Buckminsterfullerene has the formula C60.
The bonding in buckminsterfullerene is similar to the bonding in graphite.
Which of the following is true?
A All the bond angles in buckminsterfullerene are 120°.
B The melting temperature of buckminsterfullerene is higher than that of graphite.
C There are delocalized electrons in buckminsterfullerene.
D On complete combustion, buckminsterfullerene forms carbon dioxide and water.
It seems that B is the answer.
But D and A are equally right snce both will give CO2 and H2O upon combustion and all Carbon in graphite are sp2-hydridized so the bond angle will be 120....
Alterntively question will be "Which of the following is NOT true"?
Please help.
-
Originally posted by hoay:
Q. Buckminsterfullerene has the formula C60.
The bonding in buckminsterfullerene is similar to the bonding in graphite.
Which of the following is true?
A All the bond angles in buckminsterfullerene are 120°.
B The melting temperature of buckminsterfullerene is higher than that of graphite.
C There are delocalized electrons in buckminsterfullerene.
D On complete combustion, buckminsterfullerene forms carbon dioxide and water.
It seems that B is the answer.
But D and A are equally right snce both will give CO2 and H2O upon combustion and all Carbon in graphite are sp2-hydridized so the bond angle will be 120....
Alterntively question will be "Which of the following is NOT true"?
Please help.
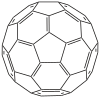
The first thing to note, is that buckminsterfullerene consists of alternating pentagons and hexagons, with 2 different bond lengths and bond angles even in the resonance hybrid (ie. not just in the resonance contributors).
A is wrong because if all C atoms had exactly 120 degrees bond angles, the molecule would be planar (like the graphene layers in graphite), but obviously buckminsterfullerene is spherical, and therefore although the C atoms are considered (largely) sp2 hybridized, the bond angles necessarily deviate from 120 degrees in order to achieve the spherical molecular geometry, which also results in molecular strain due to ring strain due to angle strain. Interestingly, although every C atom in buckminsterfullerene belongs simultaneously to both a pentagon and a hexagon, the ring strains are concentrated in the pentagons, which are non-contiguous and are required for the 'wraparound closure' of the 'graphene sheet' to form the spherical buckminsterfullerene molecule. A mathematical model of the bond angles would be 108 degrees within each pentagon, and 120 degrees within each hexagon. The actual values for the 2 different types of bond angles in the resonance hybrid of buckminsterfullerene would therefore be slightly greater than 108 degrees (within each pentagon), and slightly lesser than 120 degrees (within each hexagon), after taking resonance into consideration.
B is wrong because to melt buckminsterfullerene, you only need to partially overcome the intermolecular van der Waals attractions. To melt graphite, you need to partially overcome *both* the van der Waals attractions between the graphene layers in graphite, as well as the partial double covalent bonds within each graphene layer.
C is correct, as electrons can indeed be delocalized by across the surface of each molecule (not across molecules), due to sideways overlapping of unhybridized p orbitals of each (largely) sp2 C atom. However, the electron delocalization and aromaticity is only partial, and not complete. This is related to option A and the structure (see diagram) above. Buckminsterfullerene tends to avoid having double bonds in the pentagonal rings (ie. in the resonance hybrid, the pentagonal bond lengths and strengths have mostly single bond character, while the hexagonal bond lengths and strengths have greater partial double bond character), which makes electron delocalization poor, and results in buckminsterfullerene being only partially aromatic.
D is wrong because buckminsterfullerene isn't a hydrocarbon, but just carbon (ie. an allotrope of). Hence only product of complete combustion will be CO2 (no H2O).