Concentration problem
-
We have a 0.1 mol dm-3 solution of ethanoic acid. It was diluted with 150 cm3. What is the new concentration of the diluted solution.
Ans. When the solution was diluted the no of moles stays the same (0.1) but the volume increases (1000 + 150 = 1150 cm3 = 1.15 dm3) . The new conentration will be
0.1 / 1.15 = 0.0869 mol dm-3.
Is this the correct working??
-
Originally posted by hoay:
We have a 0.1 mol dm-3 solution of ethanoic acid. It was diluted with 150 cm3. What is the new concentration of the diluted solution.
Ans. When the solution was diluted the no of moles stays the same (0.1) but the volume increases (1000 + 150 = 1150 cm3 = 1.15 dm3) . The new conentration will be
0.1 / 1.15 = 0.0869 mol dm-3.
Is this the correct working??
Your working is correct, provided the original question is modified into "We have a 1 dm3 of 0.1 mol dm-3 solution of ethanoic acid". Your original question (without the part in bold) lacks sufficient information to answer the question. -
Well 0.1 mol dm-3 ethanoic acid means 0.1 mole ethanoic acid dissolved in 1 dm3 of water. 0.1 mol dm-3 ethanoci acid was diluted with 150 cm3. I did not understand where is the ambiguity?
-
Consider the following 2 variants on your original question. The final answer will be different for both questions.
We have a 5dm3 of 0.1 mol dm-3 solution of ethanoic acid. It was diluted with 150 cm3 of water. What is the new concentration of the diluted solution?
We have a 5cm3 of 0.1 mol dm-3 solution of ethanoic acid. It was diluted with 150 cm3 of water. What is the new concentration of the diluted solution?
Because the moles of ethanoic acid present in each case is very different, hence the final answer will also be very different. -
Distillation and fractional distillation are different in the way that fractional uses compounds having different boiling points while distillation separates impurities from a liquid. Is it correct?
-
Originally posted by hoay:
Distillation and fractional distillation are different in the way that fractional uses compounds having different boiling points while distillation separates impurities from a liquid. Is it correct?
Yes, that's correct. Simple distillation only requires you to separate and obtain 1 volatile species from a mixture containing non-volatile impurity species.
Fractional distillation is required if you wish to separate and obtain 1 volatile species (with the lower boiling point) from a mixture of 2 or more volatile species (with higher boiling points). This process may be repeated (using the same apparatus) to separate and obtain the volatile species with the next higher boiling point.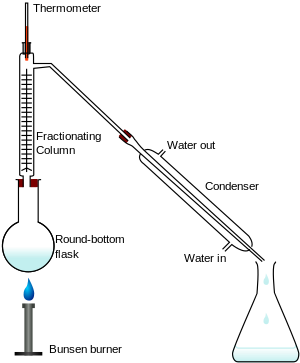
Of course, a more comprehensive, elaborate and extensive setup is used industrially, should one wish to separate and obtain all the different components of a mixture simultaneously, eg. fractional distillation of crude oil : -
If we have two alcohols like propanol and butanol then fractional distillation would separate them. Can we use chromatography to separate these alcohols? Where we will use chromatography and not fractional distillation ?
-
Originally posted by hoay:
If we have two alcohols like propanol and butanol then fractional distillation would separate them. Can we use chromatography to separate these alcohols? Where we will use chromatography and not fractional distillation ?
For O and A level purposes, the forms of chromatography used are usually more than analytical than preparative. Meaning that chromatography (for O and A level purposes, mostly paper chromatography and gel electrophoresis, ie. electrochromatographic separation of DNA / RNA / proteins / amino acids) is used for analytical purposes, ie. identifying the components of a mixture, rather than preparative, ie. to separate out large quantities of the individual components.
For O and A level purposes, fractional distillation is hence still the method of choice for preparative separation purposes. However, the limitation of fractional distillation is that it cannot be effectively used to separate non-volatile species (ie. species that are not simple covalent molecules with low boiling points), or volatile species with closely similar boiling points, or azeotropic components (an azeotrope is a mixture of two or more liquid species whose proportions remain unchanged upon distillation), from each other.
Should you be required to separate (for preparative purposes) component species from such problematic mixtures (as described above), then indeed, more advanced forms of preparative chromatography will be more suitable.
Some forms of chromatography are more suited for analytical purposes, while some forms may be adapted for preparative purposes. For a comprehensive list of advanced chromatography techniques (which are beyond the scope of the O and A level Chemistry syllabuses; but of course, Cambridge could always prove relevant information on 1 or more such advanced chromatography technique in an O or A level exam question, and query the candidate on any underlying chemical concepts which are somewhat relevant to the syllabus; as such, candidates gunning for the distinction A grade are advised to explore beyond the basic syllabus requirements for self-enrichment), see
http://en.wikipedia.org/wiki/Chromatography
http://en.wikipedia.org/wiki/Gel_electrophoresis
To answer your question about separating propanol and butanol, yes fractional distillation will suffice. -
As an application question in A level Chemistry, Cambridge has (in previous years) tested the candidate on the analytical separation of amino acids, based on their overall ionic charge (or lack thereof in zwitterionic form) in an agarose gel electrophoresis setup.
A level Chemistry students can visit Jim Clark's webpage and the relevant Wikipedia webpage to prepare themselves for such questions :
http://www.chemguide.co.uk/organicprops/aminoacids/acidbase.html
http://en.wikipedia.org/wiki/Isoelectric_focusing