Nucleophilic attack on nitriles and amides
-
Hello I have a question regarding the nucleophilic addition of nucleophiles to nitriles and amides, in particular the nucleophilic nitrogen atom of amines.
Since nitrogen is more electronegative than carbon, carbon has a partial positive charge, thus it is more prone to attack by nucleophilic attacks by nucleophile, and in the case I am asking about amines. An google search doesn't reveal much about the reaction between amines and nitriles (although i found one). Is such a reaction even possible?
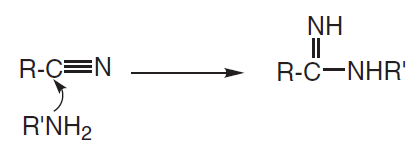
Also, is it possible that amines can attack the carbon atom of amides? There is zero information of this on the internet. This is in reference to Q27 of the 2011 h2 chem paper. What properties of amides makes it possible for amines to attack it? If so, would nitrogen gas be expelled (nucleophilic substituition) or would a amino alcohol be formed instead (nucleophilic addition, diagram shown below)?
-
Originally posted by bluepie:
Hello I have a question regarding the nucleophilic addition of nucleophiles to nitriles and amides, in particular the nucleophilic nitrogen atom of amines.
Since nitrogen is more electronegative than carbon, carbon has a partial positive charge, thus it is more prone to attack by nucleophilic attacks by nucleophile, and in the case I am asking about amines. An google search doesn't reveal much about the reaction between amines and nitriles (although i found one). Is such a reaction even possible?

Also, is it possible that amines can attack the carbon atom of amides? There is zero information of this on the internet. This is in reference to Q27 of the 2011 h2 chem paper. What properties of amides makes it possible for amines to attack it? If so, would nitrogen gas be expelled (nucleophilic substituition) or would a amino alcohol be formed instead (nucleophilic addition, diagram shown below)?

Yes to your 1st qn, nucleophilic addition unto nitrile electrophiles commonly occur, including amine nucleophiles (just that amine nucleophiles are weaker, and so this reaction doesn't occur much, so it's not in the H2 syllabus). For instance, in the hydrolysis of nitriles. In base-promoted hydrolysis, the nucleophile added onto the nitrile electrophile, is the OH- nucleophile. In acid-catalyzed hydrolysis, the nucleophile added unto the nitrile electrophile, is the H2O nucleophile (the preceeding protonation of the N atom simply increases the electron-withdrawing by induction & resonance effect, and increases the electrophilicity of the nitrile).

Yes to your 2nd qn, nucleophiles (including amines) can attack amide electrophiles (eg. water nucleophile attacking amides to hydrolyze them). However, because amides are not particularly electrophilic nucleophiles, and because amines are not particularly strong nucleophiles, the reaction does not usually proceed very far (eg. without an acid catalyst and strong heating).
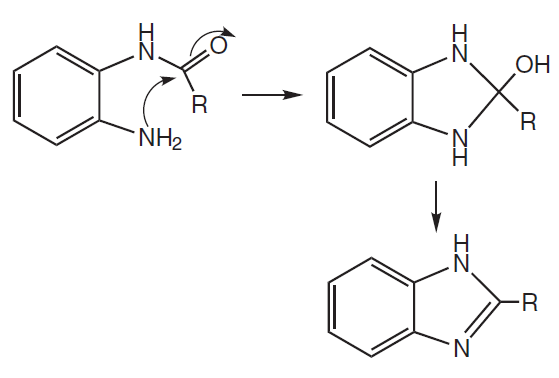
For the 2011 P1 MCQ 27, Cambridge deliberately included this (out-of-syllabus but still doable as an MCQ) qn, not because they expected H2 Chem students to be familiar with amines nucleophiles attacking amide electrophiles (on the contrary, they hoped H2 Chem students would have no idea this would happen, otherwise it would defeat the purpose of an out-of-syllabus qn), but rather that by elimination as well as plausibility consideration (ie. theoretically, any nucleophile has the potential to attack any electrophile; Cambridge was testing if the H2 Chem student at least recognized this), this slightly-beyond-syllabus MCQ could still be doable by an A-grade H2 Chem candidate (by elimination process given the other even-less-feasible options). -
Ooops, I just re-read your original qn, and realize I didn't address your 2nd qn fully.
There is no reason for nitrogen gas N2 to be expelled in your case; that only happens with (radical-nucleophilic aromatic substitution unto) diazonium electrophiles.
In your case, the pathway shown in the diagram (which differs from the Cambridge options, because this pathway might be too alien for H2 Chem students... but perhaps possibly in a future H2 Chem paper... you'd wish it comes out for your 2014 A level paper, wouldn't you? *hehe*) involves dehydration of the alpha amino alcohol to generate an imine group, with the thermodynamically favourable positive entropy change driving the reaction forward.

-
I realised my second question was incorrectly phrased and the diagram was misleading.
For the 2011 P1 MCQ Q27 Paper, should be NH3 not N2. Also, the correct diagram (after some photoshop
 ) would be this right? Amine attacks the carbonyl group of amide, after protonation and deprotonation, lonen pair on oxygen comes down to form a C=O double bond while expelling good leaving group NH3.
) would be this right? Amine attacks the carbonyl group of amide, after protonation and deprotonation, lonen pair on oxygen comes down to form a C=O double bond while expelling good leaving group NH3.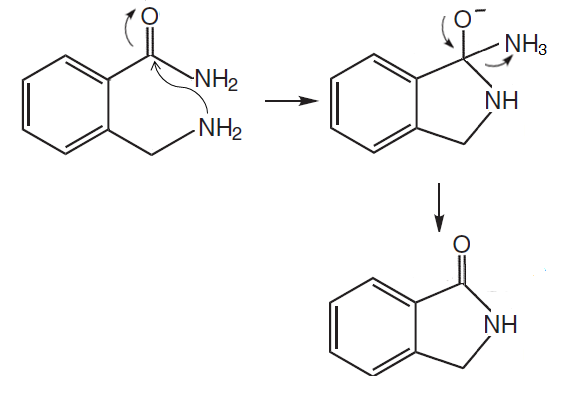
However, for this diagram, am I right to say that an amino alcohol would be formed instead of NH3 expelling , since the -NH group is directly bonded to the benzene ring hence it would be impossible for a leaving group to form.

Not sure if I am going too far out of the A level syallbus, but I have question about the nucleophilicity of the nitrogen atom in the amide group.
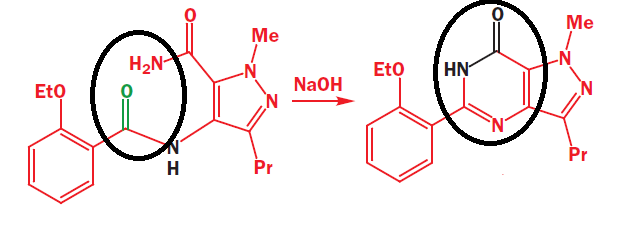
Part of the reaction scheme shows the industrial production of Sildenafil drug (viagra). Something interesting in this scheme is that a cyclic ring is formed for the product, which seems to imply that the nitrogen atom of amide must have attacked the carbonyl atom of another amide. It is really unusual, because amides are not really electrophilic, and because the lone pair of nitrogen atom in amide have delocalised into the system (since amide forms resonance, which also implies why it is neutral and not basic), the nucleophilicity of that nitrogen atom should be significantly reduced. So how did the nucleophilic attack of nitrogen atom to carbonyl group happen? What implications did the base, NaOH had on the reaction?
I know this is totally out of the A level syallbus but I'm really interested in knowing how or why such a reaction took place; really refreshing to see such unusual reactions
-
Originally posted by bluepie:
I realised my second question was incorrectly phrased and the diagram was misleading.
For the 2011 P1 MCQ Q27 Paper, should be NH3 not N2. Also, the correct diagram (after some photoshop
 ) would be this right? Amine attacks the
carbonyl group of amide, after protonation and deprotonation, lonen
pair on oxygen comes down to form a C=O double bond while expelling
good leaving group NH3.
) would be this right? Amine attacks the
carbonyl group of amide, after protonation and deprotonation, lonen
pair on oxygen comes down to form a C=O double bond while expelling
good leaving group NH3.
However, for this diagram, am I right to say that an amino alcohol would be formed instead of NH3 expelling , since the -NH group is directly bonded to the benzene ring hence it would be impossible for a leaving group to form.

Not sure if I am going too far out of the A level syallbus, but I have question about the nucleophilicity of the nitrogen atom in the amide group.

Part of the reaction scheme shows the industrial production of Sildenafil drug (viagra). Something interesting in this scheme is that a cyclic ring is formed for the product, which seems to imply that the nitrogen atom of amide must have attacked the carbonyl atom of another amide. It is really unusual, because amides are not really electrophilic, and because the lone pair of nitrogen atom in amide have delocalised into the system (since amide forms resonance, which also implies why it is neutral and not basic), the nucleophilicity of that nitrogen atom should be significantly reduced. So how did the nucleophilic attack of nitrogen atom to carbonyl group happen? What implications did the base, NaOH had on the reaction?
I know this is totally out of the A level syallbus but I'm really interested in knowing how or why such a reaction took place; really refreshing to see such unusual reactions
Yes, your mechanism for the 2011 P1 MCQ Q27 is correct.
For the amino alcohol intermediate, it's not so much that "the -NH group is directly bonded to the benzene ring hence it would be impossible for a leaving group to form", for afterall the benzene ring *is* able to stabilize the leaving group by delocalizing the negative formal charge by resonance (over its ortho and para C atoms). It's rather a matter of which pathway (it's common in chemistry, just as in real-life, to have multiple possible competing pathways) is more thermodynamically favoured, and hence, which is the major product.
Because the -NH group is bonded to the benzene ring, eliminating it does not afford as positive an entropy increase, as compared to dehydration to generate the imine functional group.
There are (of course) other variables to consider, such as the exact reagents, solvent, pH, temperature, etc. For A level H2 Chemistry purposes, it will more than suffice for the student to appreciate how both pathways are at least possible, and the Cambridge qn will be more than fair, giving hints and clues on which pathway to focus on (eg. presented as an MCQ obviously obviates the ambiguity of pathway). -
Regarding your Viagra qn, yes you're right that amides at not particularly strong nucleophiles (because the N atom's lone pair is delocalized by resonance over to the electronegative electron-withdrawing O atom), but this does not mean that the amide has completely lost all nucleophilic capacity. It simply means the Ea barrier is significantly increased. You can decrease the Ea barrier by various ways, eg. acid catalyzed or base promoted. Exactly how its done here, will depend on the reaction conditions, which must be provided by the Cambridge qn (if it were to ever appear as an A level qn).

The two (albeit weak) nucleophilic amide N atoms attack (consecutively) the carbonyl / acyl C atom electrophile, eliminating the (subsequent to proton transfers) OH leaving group as OH-, generating the ring and the imine functional group. The role of any acid or any base employed (depending on the qn), will be to facilitate proton transfers and hence lower the Ea barrier.
Lastly, yes you're right this reaction is unusual (weak nucleophile amide attacking weak electrophile amide) by A level standards, but at industrial levels there are always ways (including processes and mechanisms beyond A levels) to overcome such problems (which is why Chemical Engineers are relatively well paid... well nowhere as well paid as bankers and wall street bastards, whose existence exacerbate the Gini coefficient and leading to the downfall of humanity, but that's another topic altogether). For A levels, students should at the very least appreciate the role of temperature and positive entropy change in thermodynamically favoring certain reactions (such as in this case).