Nucleophilic Substitution-Ester formation
-
Type of reaction:Nucleophilic Substitution Reagents and Conditions:RCOOH,concentrated H2SO4,heat under reflux Sorry,I do not know how to draw the diagram via microsoft.Anyway,what is the concept behind this reaction and another reaction with acyl chloride?
-
Fischer Esterification is a nucleophilic acyl substitution reaction via the addition-elimination mechanism, and because a small molecule (eg. H2O) is removed to join two molecules together, it is also called a condensation reaction.
Because acyl halides and acid anhydrides are more reactive electrophiles compared to carboxylic acids, using acyl halides or acid anhydrides will increase both the rate (ie. kinetics) and the yield (ie. equilibrium). The reactions are similar : they are all nucleophilic acyl substitution reactions, via addition-elimination mechanisms, and are also condensation reactions.
To properly understand these reactions, you must be able to draw their mechanisms (taught to H3 Chem, Olympiad Chem and BedokFunland JC students). If you're now preparing for your A levels in week's time, don't worry too much about drawing the mechanism; rather focus on understanding it.
You can Wikipedia and Google out the mechanisms yourself. It's always a good idea to compare different sources, books and websites; it will give you a more balanced and comprehensive perspective.
-
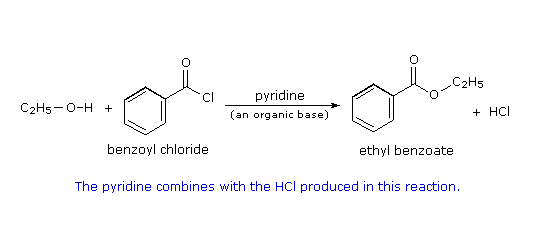
-
Why is the OH bond broken and C-Cl broken and substituted?
-
Originally posted by a mugger:
Why is the OH bond broken and C-Cl broken and substituted?
Because of the mechanism :
http://en.wikipedia.org/wiki/Nucleophilic_acyl_substitution
In the case of your qn, since you're using a neutral nucleoophile, the additional step involved is deprotonation or proton transfer (ie. the Cl- eliminated in the 2nd step, will also function as the Bronsted-Lowry base to abstract the proton from the ethanol adducted, to rid the O atom of the +ve formal charge; for A levels, it is optional whether you wish to specify the base involved in the deprotonation).
Always use the relevant mechanism to deal with Organic Chemistry questions, rather than blindly memorizing the reactions. You needn't draw out the full mechanism if the question doesn't ask for it, but at the very least, visualize or make simple sketches of the relevant mechanisms (which Cambridge examiners will ignore since the question didn't ask for it, so no worries about penalty for incomplete mechanisms done as part of your own informal working) for your own sake. It'll help you arrive at the correct answer.